3374
Combining BOLD and CBV for enhanced fMRI CNR1Radiology, University of California, San Francisco, San Francisco, CA, United States, 2San Francisco Veteran Affairs Health Care System, San Francisco, CA, United States, 3Advanced MRI Technologies, Sebastopol, CA, United States, 4Helen Wills Neuroscience Institute, University of California, Berkeley, Berkeley, CA, United States
Synopsis
In this 7T study, we investigate the constructive combination of the BOLD and CBV weighted timeseries of EPI and GRASE based Vascular Space Occupancy (VASO) sequences for enhanced fMRI CNR. Activations from this BOLD+CBV combination resulted in 9% greater t-values as well as, on average, 39% more above-threshold voxels, relative to traditional BOLD. Although this combination method resulted in reduced microvascular specificity for EPI based VASO, for GRASE based VASO, the middle layers were predominantly enhanced, suggesting minimal loss in specificity. Advantageously, this technique does not require re-acquisition of data or modifications to pulse sequence parameters of the VASO sequence.
Introduction
High-resolution fMRI at the mesoscale can facilitate improved understanding of neural circuits through experiments targeting cortical layers and columns. Recent studies have shown that cerebral blood volume (CBV) based fMRI is more specific to the actual laminar locus of neural activity than BOLD based fMRI when using standard gradient-echo (GE) echo planar imaging (EPI) sequences. However, this gain in specificity comes at the cost of roughly a factor of two in sensitivity1 (i.e. fMRI contrast-to-noise ratio; fCNR). For the Vascular Space Occupancy (VASO2,3) version of CBV-fMRI, a double image sequence is utilized giving rise to two fMRI time-series data sets acquired simultaneously: a blood nulled VASO time-series and a standard BOLD time-series. The second image is used to remove the BOLD contrast that exists in the first (blood-nulled VASO), leaving CBV contrast but at the cost of overall fCNR. We hypothesize that an optimally weighted, linear combination of the blood nulled and BOLD timeseries can result in an fMRI timeseries with enhanced fCNR, greater than BOLD alone. This should be useful for mesoscale studies of extrastriate brain regions which tend to require higher sensitivity to investigate.Methods
Two versions of VASO fMRI (one using 3D EPI and another using 3D GRASE readout) were analyzed from four healthy volunteers on a Siemens 7T MAGNETOM scanner and a 32 ch head array. During the fMRI scans, subjects were scanned once per VASO version with a 12 min finger tapping blocked task paradigm (thumb and index finger; 30 s tapping, 30 s resting). Detailed imaging parameters and post processing methods can be found in our prior publication1, and are briefly summarized here: 0.75×0.75×1.5 mm3, TR=3000 ms; FOV~100x30x12 mm3. Image volumes with blood-nulled VASO and BOLD contrast were separately corrected for motion using SPM12. Dynamic division of the blood-nulled VASO and BOLD volumes was performed to remove residual BOLD contrast contamination from the VASO images. The fCNR optimal linear combination of two z-scored timeseries of equal mean and of possibly opposite sign (e.g. our BOLD and blood-nulled timeseries) can be expressed as4: Sopt = SBOLD + raSNulled, where r = sign(corr(SBOLD,SNulled)), and a = fCNRBOLD/fCNRNulled. This combination was performed on a voxel by voxel basis on the motion corrected, preprocessed data for comparison to the original BOLD and VASO timeseries in terms of fCNR (i.e. t-test of tapping vs resting blocks) and number of voxels above a liberal threshold (t > 1, cluster size > 15).Results
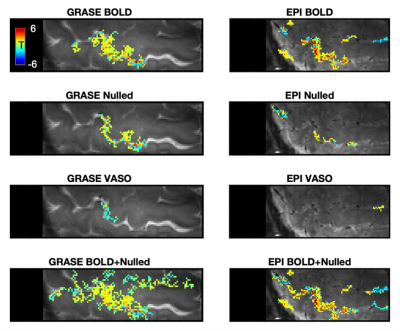
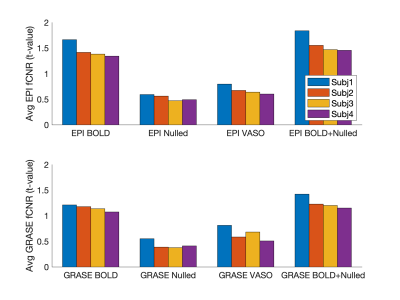
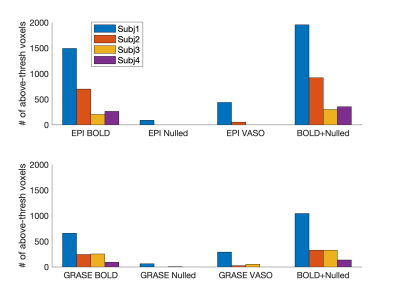
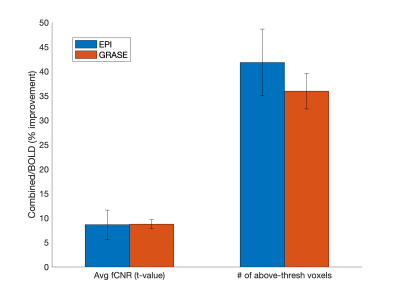
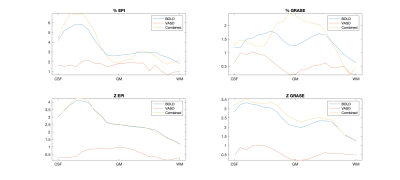
Fig. 1 shows a representative slice from one subject in terms of fMRI CNR and extent of activation (above-threshold t-values overlaid onto the timeseries mean BOLD image) for GRASE (left) and EPI (right) versions of the VASO sequence. Fig. 2 shows, per individual subject and timeseries type, the voxel average fCNR (i.e. t-value) within a unified voxel mask containing all above-threshold across the four timeseries types (e.g. BOLD, Nulled, VASO, and combined BOLD+Nulled). Fig. 3 shows, per individual subject and timeseries type, the number of above-threshold voxels. Fig. 4 shows the percent improvement, relative to the BOLD timeseries, in fCNR and number of above-threshold voxels for two versions of the VASO sequence (with SEM error bars, N=4 subjects). For the EPI and GRASE VASO sequences, the optimal combination of BOLD+Nulled significantly improved fCNR on average 9% (p<0.05, one tailed t-test) while the number of above-threshold voxels improved by 42% and 36% (respectively, p<0.05, one tailed t-test). Fig. 5 shows average motor cortex depth profiles, averaged across subjects, for signal change between superficial (toward CSF) and deep (towards WM) depths for Percent (%) Signal Change (top) and task activation Z-Score (bottom).Discussion
We found that our optimally weighted, linear combination of the blood nulled and BOLD timeseries significantly improved fMRI CNR while also expanding the spatial extent of activations relative to traditional BOLD. While Fig. 5 clearly demonstrated the expected increase in laminarly superficial, large vein bias (i.e. reduced specificity in exchange for improved sensitivity) with EPI based VASO, the combination of GRASE BOLD (which is already of high microvascular specificity relative to GE BOLD) with GRASE Nulled resulted in an increase in mostly middle layer activation which could represent an increase in sensitivity with minimal reduction of specificity. An additional benefit of this combination strategy is that it does not require re-acquisition of data or change in pulse sequence parameters of the VASO sequence. As such, this technique could be used in a complimentary nature with traditional VASO for versatile exploration of mesoscale neurocircuitry.Acknowledgements
Support: NIH BRAIN Initiative grants: 5U01EB025162, 1R24MH106096, 5R01MH111444, 1R01MH111419, 5R44NS084788.References
1. Beckett AJS, Dadakova T, Townsend J, Huber L, Park S, Feinberg DA. Comparison of BOLD and CBV using 3D EPI and 3D GRASE for cortical layerfunctional MRI at 7 T. Magn Reson Med 2020; 84(6):3128-3145.
2. Huber L, Ivanov D, Krieger SN, Streicher MN, Mildner T, Poser BA, Moller HE, Turner R. Slab-selective, BOLD-corrected VASO at 7 Tesla provides measures of cerebral blood volume reactivity with high signal-to-noise ratio. Magn Reson Med 2014;72(1):137-148.
3. Lu H, Golay X, Pekar JJ, Van Zijl PC. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn Reson Med 2003;50(2):263-274.
4. Vu A, Huth AG, Gallant J. Combining phase and magnitude information to improve source localization in complex-valued fMRI. SFN, 2010.
Figures