3373
Dependence of CBV estimated from 7T Dynamic Susceptibility Contrast data on white matter tract and cortical gray matter orientation relative to B01Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital/Harvard Medical School, Charlestown, MA, United States, 2Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Neuroimaging Research for Veterans Center, VA Boston Healthcare System, Boston, MA, United States
Synopsis
Previous studies have reported an orientation effect in DSC data within the white matter in which CBV estimates vary systematically with the orientation of local fiber orientation. Other studies have reported similar orientation effects in BOLD fMRI data both with respect to white-matter fiber orientation and gray-matter cortical orientation. Here we extend these findings by investigating orientation effects in gray and white matter in DSC-based CBV estimates. We find strong effects in both brain regions consistent with both groups of prior studies, corroborating previous interpretations that the observed BOLD effects in the white matter are vascular in origin.
Introduction
Previous studies have indirectly investigated between vascular anatomy and tissue anatomy by observing a consistent relationship between the amplitude of BOLD-fMRI signal fluctuations and the orientation of the cortical gray matter surface and the orientation of the cerebral white matter tracts relative to the B0 direction, both due to susceptibility effects[1–3]. Other studies demonstrated this orientation effect in the white matter in measurements of cerebral blood volume (CBV) from dynamic susceptibility contrast (DSC) data by comparing the estimated CBV values with local fiber orientations estimated using diffusion-weighted MRI[4–6], again most likely caused by susceptibility effects.Here we reproduce and extend these previous findings by again investigating the orientation dependence of baseline CBV estimates made from DSC data acquired at 7T in the white matter and for the first time also in the gray matter. We observe strong orientation effects in both the gray (15%) and white matter (10%), supporting previous interpretations of the vascular origins of BOLD fMRI orientation dependence in the white matter.
Methods
Twenty-nine volunteers (66±5 y.o., 17 F) participated after providing written informed consent. Volunteers were scanned on a whole-body 7-Tesla MRI scanner (MAGNETOM, Siemens). Each functional session began with two diffusion-weighted EPI protocols at b=1000 and 2000 s/mm2 (TE=62 ms, TR=5000 ms, nominal echo spacing= 0.53 ms, R=3 acceleration, no partial Fourier, 2 mm iso., 64 slices, TA=6:00) with 60 diffusion directions interspersed with seven b=0 s/mm2 images. The DSC protocol consisted of SMS-EPI[7] (TE=22 ms, TE=1500 ms, nominal echo spacing=0.69 ms, R=3, MultiBand-2, fa=75°, 58 slices, 20% gap, TA=3:08) acquired during Gadolinium contrast agent injection. Volunteers returned for an anatomical session on a 3-Tesla MRI scanner (TimTRIO, Siemens) and a Multi-Echo MPRAGE.Diffusion data were corrected for eddy current distortions and bulk motion and co-registered using the “eddy” function from FSL. The diffusion tensor model was fitted on pre-processed diffusion data using FSL's “dtifit” to derive the primary eigenvector (V1). For each subject, the averaged b=0 diffusion image was affinely co-registered to the averaged DSC image (first 10 volumes) using FSL's “flirt”. Voxel-wise V1 was then resampled to the fMRI space using the derived affine transformation with nearest-neighbor interpolation, and rotated accordingly to account for the rotation in the affine transformation.
DSC data were analyzed by first motion-correcting with FSL’s “mcflirt”, then perfusion analysis was performed with PGUI software (Center for Functionally Integrative Neuroscience, Aarhus University, Denmark) to derive parametric perfusion images. An arterial input function (AIF) from middle cerebral artery branches was selected based on cluster analysis of voxel-wise dynamic curves[8]. MTT was computed via the central volume theorem using CBV from the integration of the dynamic curve and CBF from the residue function after singular value decomposition of the AIF[9,10].
Surface reconstruction was performed automatically, using the MPRAGE data, with FreeSurfer[11].
Orientation of the cortical surface normal relative to the B0 field axis was computed for the position of the brain at the time of the DSC acquisition, as described previously[1,2]. White matter tract orientation was also computed for the position of the brain at the time of the DSC acquisition, as described previously[3]. Finally, CBV values estimated from the DSC data were binned according to both cortical gray matter and white matter orientation.
Results
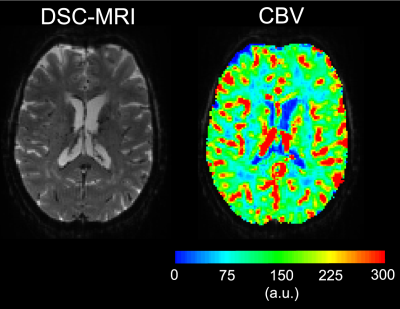
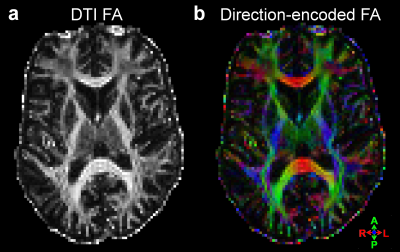
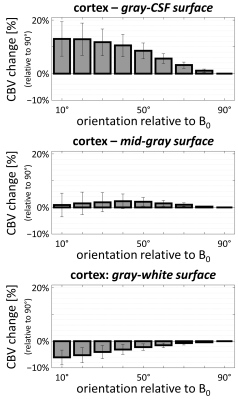
Examples of the 7T DSC data and associated CBV estimates are provided in Fig. 1, and show high data quality. Example of the same-session 7T diffusion-weighted images and associated fractional-anisotropy estimates are presented in Fig. 2.Fig. 3 presents the orientation dependence relative to the cortical surface of the CBV estimates in the gray matter for three cortical depths. At the gray/CSF interface a strong 15% effect is seen, suggesting that CBV may be overestimated in regions parallel to B0 due to strong extravascular dephasing of the pial vessels. The effect is negligible for the mid-gray surface, and reappears at the gray/white interface but with an opposite sign.
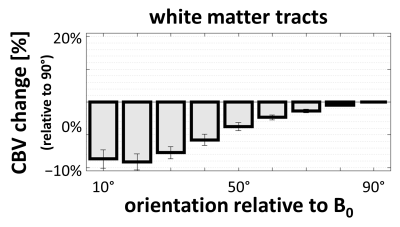
Fig. 4 presents the orientation dependence relative to the fiber tracts of the CBV estimates in the white matter. These results largely reproduce previous findings consistent with a strong effect of blood vessels running parallel to the tracts.
Discussion
Here we have reproduced previous findings both from DSC-based estimates of CBV[4–6] and from resting-state BOLD fluctuations[3] of a strong orientation dependence in the white matter that reflects the blood vessels. While our previous BOLD data exhibited WM orientation effects in part from blood vessels and presumably the myelinated fibers themselves, the current data are consistent with a strong geometric coupling between blood vessels and fiber anatomy, and support our previous interpretation of a vascular contribution to the observed BOLD orientation dependence in the white matter. We also demonstrate experimentally, for the first time, an expected orientation effect on the baseline CBV estimates in the cortical gray matter with a magnitude of roughly 15%, in this case reflecting a coupling between cortical and pial vascular geometry. These orientation biases are due to the susceptibility effect underlying the CBV estimates, which is more pronounced at higher field. Future work will quantify the strength of this orientation effect in individual fiber bundles to infer which bundles exhibit the presence of large blood vessels.Acknowledgements
We thank Kimberly Stephens and Randa Almaktoum for help with data curation and preprocessing. This work was supported in part by the NIH NIBIB (grants P41-EB030006, R01-EB019437 and R21-NS106706), by the BRAIN Initiative (NIH NIMH grants R01-MH111419 and R01-MH111438), and by the MGH/HST Athinoula A. Martinos Center for Biomedical Imaging; and was made possible by the resources provided by NIH Shared Instrumentation Grants S10-RR023043 and S10-RR019371. We gratefully acknowledge the use of Siemens Works-In-Progress (WIP) package #511.References
1. Viessmann O, Chen JE, Setsompop K, Wald LL, Polimeni JR. Variability, BOLD temporal SNR bias and variance across the HCP population as a function of cortical B0-orientation and orientation. Proc Intl Soc Mag Reson Med. 2019;27:0369.
2. Viessmann O, Scheffler K, Bianciardi M, Wald LL, Polimeni JR. Dependence of resting-state fMRI fluctuation amplitudes on cerebral cortical orientation relative to the direction of B0 and anatomical axes. Neuroimage. 2019;196:337-350. PMID: 31002965.
3. Viessmann O, Tian Q, Bernier M, Salat DH, Polimeni JR. White matter resting-state BOLD signals depend on the orientation of the local diffusion tensor axis relative to the B0-field. Proc Intl Soc Mag Reson Med. 2020;28:1367.
4. Hernández-Torres E, Kassner N, Forkert ND, Wei L, Wiggermann V, Daemen M, Machan L, Traboulsee A, Li D, Rauscher A. Anisotropic cerebral vascular architecture causes orientation dependency in cerebral blood flow and volume measured with dynamic susceptibility contrast magnetic resonance imaging. J Cereb Blood Flow Metab. 2017;37(3):1108-1119. PMID: 27259344.
5. Kaczmarz S, Göttler J, Zimmer C, Hyder F, Preibisch C. Characterizing white matter fiber orientation effects on multi-parametric quantitative BOLD assessment of oxygen extraction fraction. J Cereb Blood Flow Metab. 2020;40(4):760-774. PMID: 30952200.
6. Doucette J, Wei L, Hernández-Torres E, Kames C, Forkert ND, Aamand R, Lund TE, Hansen B, Rauscher A. Rapid solution of the Bloch-Torrey equation in anisotropic tissue: Application to dynamic susceptibility contrast MRI of cerebral white matter. Neuroimage. 2019;185:198-207. PMID: 30332614.
7. Setsompop K, Gagoski BA, Polimeni JR, Witzel T, Wedeen VJ, Wald LL. Blipped-controlled aliasing in parallel imaging for simultaneous multislice echo planar imaging with reduced g-factor penalty. Magn Reson Med. 2012;67(5):1210-1224. PMID: 21858868.
8. Mouridsen K, Christensen S, Gyldensted L, Østergaard L. Automatic selection of arterial input function using cluster analysis. Magn Reson Med. 2006;55(3):524-531. PMID: 16453314.
9. Mouridsen K, Friston K, Hjort N, Gyldensted L, Østergaard L, Kiebel S. Bayesian estimation of cerebral perfusion using a physiological model of microvasculature. Neuroimage. 2006;33(2):570-579. PMID: 16971140.
10. Jespersen SN, Ostergaard L. The roles of cerebral blood flow, capillary transit time heterogeneity, and oxygen tension in brain oxygenation and metabolism. J Cereb Blood Flow Metab. 2012;32(2):264-277. PMID: 22044867.
11. Fischl B. FreeSurfer. Neuroimage. 2012;62(2):774-781. PMID: 22248573.
Figures