3372
Multi-centre, multi-vendor 7 Tesla fMRI reproducibility of hand digit representation in the human somatosensory cortex1Cardiff University Brain Research Imaging Centre, School of Psychology, Cardiff University, Cardiff, United Kingdom, 2Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom, 3Wellcome Centre for Integrative Neuroimaging, University of Oxford, Oxford, United Kingdom, 4Wolfson Brain Imaging Centre, Department of Clinical Neurosciences, University of Cambridge, Cambridge, United Kingdom, 5Imaging Centre of Excellence, University of Glasgow, Glasgow, United Kingdom, 6Department of Neuroscience, Imaging and Clinical Sciences, "G. D’Annunzio University" of Chieti-Pescara, Chieti, Italy, 7Institute for Advanced Biomedical Technologies, "G. D’Annunzio University" of Chieti-Pescara, Chieti, Italy
Synopsis
This study compares within- and across-site reproducibility of 7 Tesla somatomotor fMRI using a travelling wave task to map the hand and individual digit localization at high spatial resolution. We performed a “travelling-heads” study acquiring data on Siemens Magnetom, Siemens Terra and Philips Achieva whole-body 7 Tesla MRI systems at five sites. Simple harmonization of the EPI sequence protocol was performed. We show metrics of intra- and inter-site variability of digit representations, with good reproducibility observed across sites. These results demonstrate the potential benefits of multi-site 7T fMRI studies for digit mapping where large cohorts are required.
Introduction
Whilst considerable progress has been made in using ultra-high field fMRI to study brain function at fine spatial resolution1-4, methods are generally optimized at a single site and do not translate to studies where multiple sites are required for sufficient subject recruitment. With a recent increase in installations of human 7 T systems, there is now the opportunity to establish a framework for multi-site 7 T fMRI studies. However, an understanding of the inter-site variability of fMRI measurements is required for datasets to be combined across sites. To address this, we employ a hand digit localization task5,6 and compare across-site and within-site reproducibility of 7 T fMRI to a hand digit localization task which requires fine spatial resolution to resolve individual digit representations.Methods
Ten healthy subjects (32±6 years; 3 female; 1 left-handed) participated in the study. To assess inter-site repeatability, the same protocol was repeated at five sites, using three different 7 Tesla whole-body MRI systems (Site 1: Siemens Terra; Site 2: Siemens Magnetom; Site 3: Siemens Terra; Site 4: Philips Achieva; Site 5: Siemens Magnetom). The same model of volume-transmit, 32-channel receive head coil (Nova Medical) was used at each site. Subjects were split into five pairs, with each pair returning to one site an additional four times (two subjects per site), to assess intra-site repeatability.Two runs of a visually-cued, travelling wave somatomotor task were performed with the dominant hand. This entailed a visually paced sequential 1 Hz button press of 8 s blocks of digit movement, cycling across digit blocks from index finger (D2) to little finger (D5) in a “forward” run and from D5 to D2 in a “reverse” run (32s per four digits), with 8 cycles per run. Gradient-echo EPI protocol: 1.5mm isotropic resolution, TR=2s, TE=25ms, echo spacing 0.68/0.78ms Siemens/Philips, 34/28 slices Siemens/Philips. Spin-echo EPI scans were acquired with matched and reversed phase-encode direction7 for distortion correction. 2D FLASH (0.75x0.75x1.5mm3, TE=10ms, TR=1100ms) and MPRAGE (0.7mm isotropic, TR/TE/TI=2200/3.05/1050ms, FA=7°) datasets were acquired for realignment and cortical flattening.
Data was motion corrected (FSL MCFLIRT8), distortion corrected (FSL TOPUP9), and temporal filtered (100s cut-off) using FSL FEAT10. A Fourier-based travelling wave analysis was performed using mrTools11 to calculate voxel-wise phase and coherence of the BOLD response. Image registration was performed using mrTools11. Whole-hand activation regions (FDR-corrected p < 0.05) were compared across sessions using Dice’s overlap coefficient (Dice). An intersection mask was formed for each subject by including voxels identified as active across all five sites. Individual digit maps were formed within this intersection mask by dividing the phase maps into four equal π/2 portions. Digit overlap across sessions was compared using the Dice similarity coefficient. Intra- and inter-site reproducibility were compared using Bonferroni-corrected paired t-tests applied to the mean Dice coefficient across repeated sessions in the same site and across sites, respectively.
Results
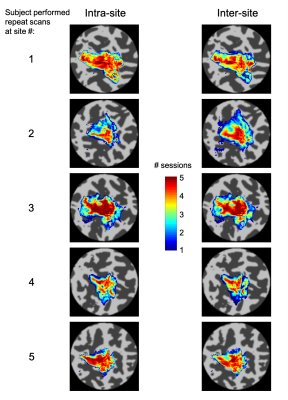
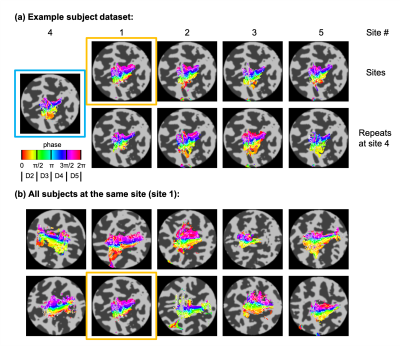
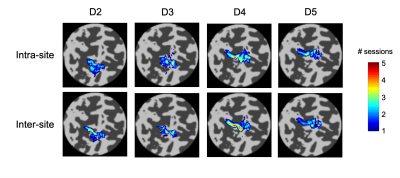
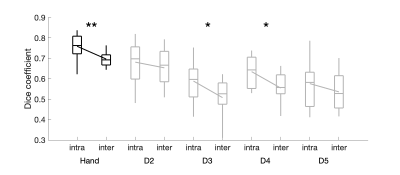
Temporal SNR was similar across the five sites (tSNR = 45±4/43±4/46±5/45±4/44±3; F(4,45)=0.89, p = 0.48). Figure 1 presents example conjunction maps for five subjects, showing the number of sessions with overlapping whole-hand activation regions, across repeats at the same site (intra-site), or across sites (inter-site), these datasets were chosen to present intra-site data from each of the five sites. Phase (digit) maps are shown in Figure 2, with all sessions from a single subject shown (Fig. 2a) and all ten subjects’ phase maps for a single site (Fig. 2b). The spatial distribution of the digit representations are consistent across sites for a single subject, whereas there is a high degree of inter-subject variability. Figure 3 shows both intra-site and inter-site conjunction maps for each digit, with a high degree of similarity between intra-site and inter-site for individual digits. Dice coefficients for the whole hand region are higher for intra-session measures from the individual sites compared to inter-site measures (Figure 4; t(9) = -5, pcorr = 0.002). There is also a similar trend in the individual digit maps for intra-site Dice coefficients to be greater than inter-site (Fig. 4), but this only survives Bonferroni correction (pcorr<0.05) in D3 and D4.Discussion
This study demonstrates good reproducibility of fMRI digit maps across five, 7 T sites, using three different models of whole-body MRI system from two manufacturers. The 1.5 mm isotropic spatial resolution fMRI data acquired here was able to resolve inter-subject differences in hand digit representations, consistent with previous observations6. Simple protocol harmonization, such as matching EPI echo train duration, in-plane acceleration factor, acquisition matrix, TE and TR, appears to be sufficient to achieve good reproducibility across MRI systems. We did not harmonize the image reconstruction, coil combination, or parallel acquisition methods. We show that the inter-site Dice overlap was smaller than intra-site. However, this difference in Dice coefficients corresponds to ~5% difference in overlap in the regions between intra- and inter-site comparisons, which is small compared to the inter-session variability. Inter-site Dice values are similar to those reported across sessions in an intra-site study6.Conclusion
High resolution fMRI studies can be performed across multiple sites, with inter-site factors contributing. The inter-site variability measured here, in the form of Dice coefficients, can be used to inform future study designs and sample size calculations for multi-site somatomotor mapping studies.Acknowledgements
We acknowledge the UK Medical Research Council for funding support (MR/N008537/1). CTR is funded by the Wellcome Trust and the Royal Society [098436/Z/12/B]. CR is funded by the Cambridge Centre for Parkinson-plus.References
1. Benson NC, Jamison KW, Arcaro MJ, et al. The Human Connectome Project 7 Tesla retinotopy dataset: Description and population receptive field analysis. J Vis. 2018; 18(13):1–22.
2. Puckett AM, Bollmann S, Barth M, Cunnington R. Measuring the effects of attention to individual fingertips in somatosensory cortex using ultra-high field (7T) fMRI. Neuroimage. 2017; 161:179–87.
3. Huber L, Finn ES, Handwerker DA, et al. Sub-millimeter fMRI reveals multiple topographical digit representations that form action maps in human motor cortex. Neuroimage. 2020; 208:116463.
4. Van der Zwaag W, Kusters R, Magill A, et al. Digit somatotopy in the human cerebellum: A 7T fMRI study. Neuroimage. 2013; 67:354-362.
5. Sanchez-Panchuelo RM, Francis S, Bowtell R, Schluppeck D. Mapping human somatosensory cortex in individual subjects with 7T functional MRI. J Neurophysiol. 2010; 103(5):2544–56.
6. Kolasinski J, Makin TR, Jbabdi S, et al. Investigating the stability of fine-grain digit somatotopy in individual human participants. J Neurosci. 2016; 36(4):1113–27.
7. Driver ID, Mougin O, Clarke WT, et al. A comparison of distortion correction methods for EPI fMRI applied across three 7T platforms. Proc. ISMRM 2018; 26: 5464.
8. Jenkinson, M., Bannister, P., Brady, J. M. et al. Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002; 17(2), 825-841.
9. Andersson J, Skare S and Ashburner B. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. NeuroImage. 2003; 20:870-888.
10. Smith S, Jenkinson M, Woolrich M et al. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23(S1):208-219.
11. Gardner, JL, Merriam, EP, Schluppeck, D, Besle, J, & Heeger, DJ (2018). mrTools: Analysis and visualization package for functional magnetic resonance imaging data (Version 4.7). Zenodo. http://doi.org/10.5281/zenodo.1299483
Figures