3369
Layer-specific activation of prediction in the human midcingulate cortex1Graduate School of Interdisciplinary Science and Engineering in Health Systems, Okayama University, Okayama, Japan, 2Section on Functional Imaging Methods, National Institute of Mental Health, Bethesda, MD, United States, 3Division of Cerebral Integration, National Institute for Physiological Sciences, Okazaki, Japan, 4Center for Information and Neural Networks, National Institute of Information and Communications Technology, Suita, Japan, 5Department of Cognitive Neuroscience, Maastricht University, Maastricht, Netherlands
Synopsis
The human brain is constantly generating and updating predictions. The midcingulate cortex (MCC) is one of the areas that contribute to prediction processing, while MCC layer-specific function remains unknown. In the present study, we used a tactile index finger prediction task that consists of sequential finger poking during high-resolution (0.76mm) BOLD and VASO fMRI at 7T to investigate how the prediction activity changes across layers in the human MCC. We found the double-peak activity feature across MCC layers for all prediction tasks, and the enhanced activity within MCC superficial layers may reflect the prediction error processing.
BACKGROUND
The prediction processing is achieved within bidirectional hierarchically organized cortical structures in the human brain(1). The higher-level areas was known to generate predictions and transmit signals back to lower-level areas (i.e., primary sensory cortex)(2). In contrast, the lower-level areas serve to calculated prediction errors between the perceived sensory input and prediction and then transmit error signals back to higher-level areas. In our previous studies(3, 4), we found that both the prediction and prediction error processing yielded activity in the superficial and deep layers of the human primary somatosensory cortex (S1). However, the precise contribution of higher-level area layers to prediction processing has not been investigated to date. In the present study, we focused on the human midcingulate cortex (MCC), one of the higher-level areas contributing to prediction processing, to investigate layer-specific activity related to tactile prediction. We acquired high-resolution fMRI at 7T(3, 5) and sought to identify layer-specific activity in MCC by using a series of index finger prediction tasks.METHODS
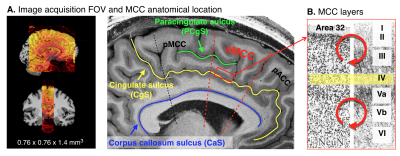
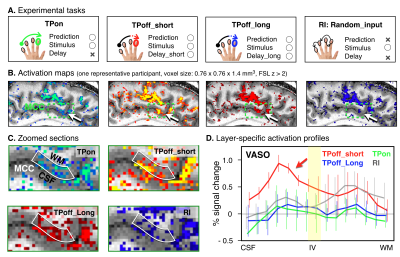
Four participants were asked to participate in the fMRI experiment to investigate the cortical layer-specific brain responses reflecting the prediction activity. All participants provided written informed consent prior to their participation in the study. The study protocol was approved by the local medical ethics committee at the Okayama University Hospital and National Institute for Physiological Sciences. A 7T Siemens scanner, equipped with a 32-channel NOVA Medical head coil and SC72 body gradient coil was used. The MCC is located caudally in the anterior cingulate gyrus of the limbic lobe, and it is a dysgranular area that does not have a well-developed layer IV (Figure 1A&B). The layer-specific fMRI data acquisition procedures were used as described in our previous study(5). The coil-combined data consist of interleaved BOLD and blood volume sensitive VASO contrasts – obtained as separate yet concomitant time series. These time series are corrected for rigid volume motion and are separated by contrast with an effective temporal resolution of TR = 4.4 s. The nominal resolution of the 3D-EPI readout(6) was 0.76 mm across cortical depths with 1.4-mm thick slices (Figure 1A). After the fMRI acquisition, a T1-weighted high-resolution anatomical whole brain image (voxel size = 0.7 mm isotropic) and slab-selective high-resolution (0.5 mm × 0.5 mm × 1.4 mm) anatomical data covering the MCC were collected by the MP2RAGE SIEMENS prototype sequence. As shown in Figure 2A, we designed three tactile temporal prediction (TP) tasks and one control task with random input (RI). In the TP on-beat (TPon) task, the temporal rhythm of index finger poking matched the predicted rhythm learned from isochronous stroking of the first three fingers (i.e., the interval between each stimulus was constant). Alternatively, in the other two TP off-beat (TPoff) tasks, the temporal rhythm to the index finger did not match the predicted timing. Rather, isochronous stimulation of the first three fingers was followed by index finger stimulation with a variable delay either of one-beat interval (additional 0.37 s, termed the TPoff_short task) or two-beat intervals (additional 0.75 s, termed the TPoff_long task). The participant was asked to predict when the left index finger will be stroked in three TP tasks, however, they were asked to simply pay attention to the stimulation without any prediction involved in RI task. Laminar analyses were conducted with the open software suite LayNii(7).RESULTS
TP task-induced fMRI signal change in the MCC was found in all participants. As shown in Figure 2B&C, layer-specific activity for BOLD-fMRI modulations across tasks could be detected in a representative participant’s individual activation maps. Averaged profiles of layer-specific VASO responses for the four tasks are shown in Figure 2D. We found the double-peak activity feature across MCC layers for all tasks and TPoff_short task enhanced activity within the superficial layers than all other tasks.DISCUSSION
In the present study, our main finding is that both superficial and deep layers of MCC play a crucial role in the prediction processing. By comparing layer-specific activity in MCC during the three TP tasks, we showed that activity in superficial layers is selectively modulated by the error processing which occurred during the delayed period of the TPoff_short. In line with the predictive coding principle(8), the more robust activation in superficial layers may reflect the prediction updating or error correction processing. However, we found that the increased superficial layer activity in the TPoff_short task was temporally turned by extending the off-beat interval in the TPoff_long task. One possible interpretation is that somewhat inhibitory processing may predominate during the longer delay period, while much more work is necessary to obtain a comprehensive understanding of the all underlying processes.CONCLUSION
We used sub-millimeter BOLD and VASO fMRI at 7T to demonstrate that the superficial and deep layers of human MCC play a crucial role in the prediction processing. This laminar specificity was directly visible on functional MRI maps during task-based activity changes. Our findings provide insights into how these laminar circuits represent the prediction error details and we regard these finding as an important step towards the understanding of predictive coding processing dynamics.Acknowledgements
We thank Prof. Benedikt Poser for sharing the 3D-EPI readout sequence, Dr. Tobias Kober and Dr. Bénédicte Maréchalfor providing the MP2RAGE sequence used here. This work was supported by JSPS KAKENHI Grant Numbers JP20K07722, JP18K15339 and Japan-U.S. Science and Technology Cooperation Program (Brain Research), and Cooperative Study Program (20-635) of National Institute for Physiological Sciences.References
1. Bastos AM, Usrey WM, Adams RA, Mangun GR, Fries P, Friston KJ: Canonical Microcircuits for Predictive Coding. Neuron 2012; 76:695–711.
2. Shackman AJ, Salomons T V., Slagter HA, Fox AS, Winter JJ, Davidson RJ: The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci 2011; 12:154–167.
3. Yu Y, Huber L, Yang J, et al.: Layer-specific activation of sensory input and predictive feedback in the human primary somatosensory cortex. Sci Adv 2019; 5:1–10.
4. Yu Y, Huber L, Chai Y, et al.: Increased activity in superficial and deep layers of human S1 for temporal prediction error. In Proc Intl Soc Mag Reson Med 27; 2019; 613.
5. Huber L, Handwerker DA, Jangraw DC, et al.: High-Resolution CBV-fMRI Allows Mapping of Laminar Activity and Connectivity of Cortical Input and Output in Human M1. Neuron 2017; 96:1253–1263.
6. Poser BA, Koopmans PJ, Witzel T, Wald LL, Barth M: Three dimensional echo-planar imaging at 7 Tesla. Neuroimage 2010; 51:261–266.
7. Huber L (Renzo), Poser BA, Bandettini PA, et al.: LayNii: A software suite for layer-fMRI. bioRxiv 2020:1–20.
8. Seth AK, Friston KJ: Active interoceptive inference and the emotional brain. Philos Trans R Soc B Biol Sci 2016; 371:20160007.
Figures