3366
Motor preparatory inhibition is reflected as a layer dependent suppression in the human primary motor cortex
Yinghua Yu1,2, Ikuhiro Kida1,2, and Nobuhiro Hagura1,2
1Center for Information and Neural Networks, National Institute of Information and Communications Technology, Osaka, Japan, 2Graduate School of Frontier Biosciences, Osaka University, Osaka, Japan
1Center for Information and Neural Networks, National Institute of Information and Communications Technology, Osaka, Japan, 2Graduate School of Frontier Biosciences, Osaka University, Osaka, Japan
Synopsis
In humans, corticospinal excitability is reduced when preparing for an action; i.e. motor preparatory inhibition. Functional relevance of this inhibition has been proposed, however, the site where the actual inhibition occurs, either at the primary motor cortex (M1) or at the downstream spinal-circuit, is still unclear. By measuring vascular space occupancy (VASO) with 7T fMRI, we investigated the direct signature of preparatory inhibition of neural activity across M1 cortical layers. Our preliminary analysis showed that preparation was associated with a reduced activity of the superficial layers of M1. Motor preparatory inhibition likely takes place in M1 in a layer-dependent manner.
Introduction
In humans, corticospinal excitability is reported to be reduced during motor preparation, which is termed as motor preparatory inhibition 1,2. Functional relevance of such inhibition has been proposed 1,2, however, in humans, direct evidence for the site where the neural activity is inhibited is still lacking. This is because the motor preparatory inhibition is mainly identified by measuring the motor evoked potential (MEP) elicited by transcranial magnetic stimulation (TMS), which can reflect either the inhibition at the cortical stimulation site (primary motor cortex; M1) or at the downstream spinal level. Here, by using 7T fMRI and measuring vascular space occupancy (VASO) across M1 cortical layers during reaction time tasks, we investigated the layer-dependent neural signature of the motor preparatory inhibition.Methods
Five participants volunteered in a two-hour scanning session using a 7T MRI scanner (Magneton, Siemens Healthcare, Erlangen, Germany) equipped with a 32-channel head coil (Nova Medical, Wilmington, MA, USA) and SC72 body gradient coil. Data acquisition procedures were same as previously literature 3,4. In short: a multi-contrast VASO-BOLD sequence with slices was positioned to be approximately perpendicular to the central sulcus of the left hemisphere. The coil-combined data consist of interleaved BOLD and blood volume sensitive VASO 5 contrasts – obtained as separate yet concomitant time series. The parameters of the acquisitions were: Nominal in-plane resolution = 0.75mm, TE/TI1/TI2/TR = 24/900/2548/3296ms, ten slices with FLASH GRAPPA-2, matrix 44x132, 3D-EPI readout, FOV 31.2x93.7x21.6 mm3.As for the task, participants pressed a button by flexing their right wrist, in response to a visual imperative stimulus (green circle) presented on the screen. Three conditions, each assigned to different runs, were prepared. In the M (movement) condition, imperative stimulus was presented with random inter-stimulus-intervals. In the PM (prepared movement) condition, 500ms prior to every imperative stimulus, a warning signal (red circle) was presented. In the PM & M condition, half of the imperative stimulus was associated with the warning signal, but the other half was not. Each run consisted of 12 mini-blocks, with each 36 second mini-block separated with 30 seconds rest. If motor preparatory inhibition takes place in the M1, PM condition that contains motor preparatory period should have the lowest activity compared to the other two conditions. Laminar analyses were conducted with the open software suite LayNii 6.Results
Reaction times were faster when the Warning signal was present (5 out of 5 participants), indicating that the Warning signal worked as to facilitate the subsequent motor output (Figure 1B). Functional MRI signal changes, both for BOLD and CBV, was consistently found in the ‘hand-knob’ area of the precentral gyrus, which is the anatomical location corresponding to the hand area of M1. As shown in Figure 1A, layer-dependent activity for BOLD-fMRI modulations across tasks could be detected in the individual activation maps (representative participant). Averaged profiles of layer-dependent BOLD and VASO responses for the three task conditions are shown in Figure 1C. The functional activity showed increased response in the superficial and deep layers in all conditions; however, particularly for the VASO response, the signal was weakest for the PM condition compared to the M condition (Figure 1C. This pattern was most consistent in the superficial layers of M1 (5 out of 5 participants).Discussion & Conclusion
Using sub-millimeter BOLD and blood-volume-sensitive (VASO) fMRI in the human motor cortex, we found the reduction of neural activity when the Warning signal facilitated the response to the imperative cue. This preliminary result resembles the pattern of motor preparatory inhibition, thus, indicating that the previously reported preparatory inhibition reflects the computation at the supraspinal level (M1). Indeed, our observation fits with the recently reported two-photon imaging study in mice, where neurons in the superficial layers are selectively suppressed during a delayed reaching task 7. Such inhibitory cortical input to M1 may contribute to the preparation of the selected motor command to be executed efficiently, making the system robust to the interference from other possible motor programs.Acknowledgements
We thank Dr. Laurentius Huber and Prof. Benedikt Poser for their contribution to the MR-sequence. This study was partly supported by Japan Society for the Promotion of Science (Kakenhi 26119535, 18H01106) to NH.References
- Duque J, Greenhouse I, Labruna L, Ivry RB. Physiological Markers of Motor Inhibition during Human Behavior. Trends Neurosci. 2017, 40: 219-236
- Hannah R, Cavanagh SE, Tremblay S, Simeoni S, Rothwell JC. Selective Suppression of Local Interneuron Circuits in Human Motor Cortex Contributes to Movement Preparation. J Neurosci. 2018, 38:1264-1276.
- Huber L, Handwerker DA, Jangraw DC, Chen G, Hall A, Stüber C, Gonzalez-Castillo J, Ivanov D, Marrett S, Guidi M, Goense J, Poser BA, Bandettini PA. High-Resolution CBV-fMRI Allows Mapping of Laminar Activity and Connectivity of Cortical Input and Output in Human M1. Neuron 2017, 1253–1263
- Yu Y, Huber L, Yang J, et al.: Layer-specific activation of sensory input and predictive feedback in the human primary somatosensory cortex. Sci Adv 2019; 5:1–10.
- Lu H, Golay X, Pekar JJ, van Zijl PCM. Functional magnetic resonance imaging based on changes in vascular space occupancy. Magn. Reson. Med. 2003, 50: 263–274
- Huber L (Renzo), Poser BA, Bandettini PA, et al.: LAYNII: A software suite for layer-fMRI. bioRxiv 2020:1–20
- Hasegawa M, Majima K, Itokazu T, Maki T, Albrecht UR, Castner N, Izumo M, SohyaK, SatoTK, KamitaniY, SatoTR (2017) Selectivesuppression of local circuits during movement preparation in the mouse motor cortex. Cell Rep 18:2676 –2686
Figures
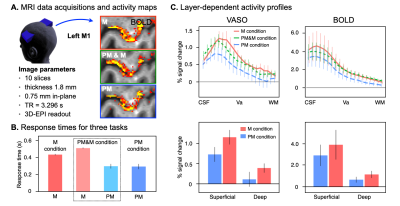
Figure 1 (A) BOLD signal activity maps from a representative participant and MRI data acquisition parameters. (B) Averaged (n = 5) response times for three task conditions. (C) Upper panel; Cortical profiles of VASO and BOLD activity changes in the hand-knob area. Lower panel; Activity changes averaged within superficial and deep layers. Error bars indicate the standard error of means across participants. M, Movement; PM, Prepared Movement.