3358
Reaching low-gadolinium concentration detection through sequences optimization on a dedicated phantom1Hospital Foundation A. de Rothschild, Imaging department, Paris, France, PARIS, France, 2Sorbonne University, Paris Brain Institute, Paris, France, Paris, France, 3Hospital Foundation Rothschild,Neurology department, Paris, France, Paris, France, 4Hospital Foundation A. de Rothschild, Pharmaceutical department, Paris, France, PARIS, France
Synopsis
Gadolinium-enhanced imaging provides valuable information in clinical practice for the diagnosis of several diseases. Conventional sequences fail to detect low concentrations of gadolinium, such as those encountered in abnormal meninges. We designed a phantom containing 16 diluted gadobutrol tubes to compare and optimize sequences, based on normalized signal and contrast-to-noise ratio, for each gadolinium concentration. We performed a preliminary study comparing T1, FLAIR and FLAIR with optimized parameters on this phantom. The optimized FLAIR sequence shows an efficient detection of low concentrations, with a higher CNR than other sequences. Preliminary in-vivo experiment shows promising results for leptomeningeal abnomalities detection.
Introduction
Gadolinium-based contrast agents provide valuable information in clinical practice, and intravenous injection before or during MR imaging is often required. Indeed, many CNS disorders, such as Multiple Sclerosis (MS) [1], require intravenous injection of gadolinium (Gd) to be detected or assessed. However, due to recent evidences of gadolinium deposition in the brain [2], clinical strategies to reduce lifespan exposure to gadolinium in patients requiring repeated scans are developed. Among them, developing sequences with improved sensitivity to lower gadolinium concentrations would allow to reduce the required injected dose to reach the diagnosis. It is proven that 3-dimensional fluid-attenuated inversion recovery (FLAIR) has a higher sensitivity than T1-weighted (T1-w) imaging to detect low concentrations of Gd. In MS, this sequence is efficient to detect leptomeningeal enhancement from extravasation of gadolinium in the meninges [3], [4]. However, in some patients, meningeal Gd concentration is too low to be detected with FLAIR sequences. The modification of refocusing angle schemes might reduce the signal of the normal brain parenchyma, and increase the signal of small gadolinium concentration contents in sulci. This project aims to evaluate whether the optimized FLAIR could detect lower dose of Gd than classical FLAIR and T1w sequences after intravenous Gd administration.Methods
Phantom studyWe designed a phantom using 16 conical tubes of 50mL filled with diluted gadobutrol and saline, in a 1% agarose gel solution in a 5 L plastic container (Figure 1). The original Gd (1.0mmol/mL solution) was diluted with saline to a reference concentration at 1.152mg/ml that simulate the concentration of gadolinium in the brain CSF, within the first 8 hours after intravenous injection. Dilutions range from x3 to 1/2 000 000 of the theoretical concentration of the extravasated gadolinium. The phantom was scanned on a 3-tesla MR system (Ingenia, Philips) using a 32-channel array head coil. Imaging protocol included the following sequences (Figure 2A-D): T1-w TFE (TR/TE=8.0/3.5ms, resolution = 1x1x1mm3), T1 TSE (TR/TE=500/27ms, resolution = 1x1x1mm3), FLAIR (TR/TE/TI=8000/400/2400ms, resolution = 1x1x1mm3), and an optimized FLAIR with the same MR parameters than conventional FLAIR but a modified refocusing angles scheme. Each tube was segmented and labeled using an in-house tool on the T1 TFE sequence (Figure 2E), and the mean and standard deviation of MR signal was measured over 6 continuous slices. The mean signal of each tube was then normalized to the saline tube. Contrast-to-noise ratio (CNR) was computed between saline and fluid with diluted Gd, defining noise as the standard deviation (SD) of the region of interest (ROI) in the saline tube (SDsaline). We calculated CNR as the difference between the signal intensity of the Gd-containing tubes (SIGd) and saline (SIsaline), divided by SDsaline from, as follow: $$CNR = | SIGd-SIsaline|/SDsaline$$.
In-vivo study
As all sequences performed on the phantom are part of the clinical routine for MS follow-up, a qualitative evaluation was performed on a patient with MS. The clinical follow-up includes similar sequences to phantom MR protocol: T1 TSE, FLAIR and optimized FLAIR.
Results
Phantom studyFigure 3A shows the normalized mean signal of the three sequences from six slices in each diluted solution. In all sequences, the normalized mean signal globally increases with increasing concentration of gadolinium. For gadolinium doses inferior at 1/10, the optimized FLAIR has a higher normalized mean signal compared to the T1 TSE sequence, relative to the saline reference. The optimized FLAIR has a higher normalized mean signal than the conventional FLAIR for doses superior to 1/100. However, while the optimized FLAIR has not a higher normalized mean signal for very low gadolinium concentration (<1/100), the CNR of the optimized FLAIR is higher for low dose (>1/100; mean CNRoptimized FLAIR = 1.86, CNRFLAIR=1.24, respectively) and as good as the one of the conventional FLAIR for very low dose (>1/1 000 000; CNRoptimized FLAIR = 0.47, CNRFLAIR=0.24, respectively).
In-vivo study: preliminary results
Following results on the phantom, suggesting that the optimized FLAIR might be effective to detect low doses of extravagated Gd, we reviewed sequences from a patient with MS (female, 31Y) on whom the 3 sequences have been performed. A linear leptomeningeal enhancement was visible (Figure 4) only visible on the optimized FLAIR, due to both brain and cerebrospinal fluid suppression.
Discussion
In this study, we present a customized phantom that can be used to assess the performances of various sequences and parameters to detect low-Gd concentrations. We use that tool to demonstrate that the FLAIR sequence can be made more sensitive to subtle gadolinium enhancements by modifying refocusing angle schemes. While signal intensity decreases with decreasing Gd concentration for all sequences, optimized FLAIR sequence offers a higher CNR than other sequences showing its interest to detect very low doses of Gd (expressed as mg/ml). This higher CNR might improve the sensitivity to detect subtle extravagated Gd in the meninges, which is highlighted in several pathologies, such as Multiple Sclerosis. However, the drop of signal for high concentration that is visible for IR sequence might be due to suboptimal MR parameters, and some new experiments are needed varying the repetition time (and its corresponding inversion time) or the refocusing angle schemes, to fully describe the signal evolution of the optimized FLAIR sequence.Acknowledgements
No acknowledgement found.References
[1] A. J. Thompson et al., « Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria », Lancet Neurol., vol. 17, no 2, p. 162‑173, févr. 2018, doi: 10.1016/S1474-4422(17)30470-2.
[2] R. J. McDonald et al., « Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging », Radiology, vol. 275, no 3, p. 772‑782, mars 2015, doi: 10.1148/radiol.15150025.
[3] P. Eisele et al., « Investigation of leptomeningeal enhancement in MS: a postcontrast FLAIR MRI study », Neurology, vol. 84, no 8, p. 770‑775, févr. 2015, doi: 10.1212/WNL.0000000000001286.
[4] M. Absinta et al., « Gadolinium-based MRI characterization of leptomeningeal inflammation in multiple sclerosis », Neurology, vol. 85, no 1, p. 18‑28, juill. 2015, doi: 10.1212/WNL.0000000000001587.
[5] F. Berger et al., « Gadolinium distribution in cerebrospinal fluid after administration of a gadolinium-based MR contrast agent in humans », Radiology, vol. 288, no 3, p. 703–709, 2018.
Figures
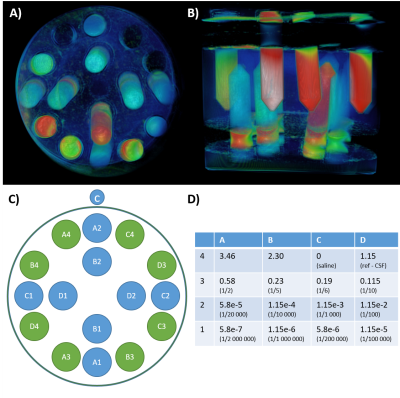
Figure 1: Phantom design.
We prepared a phantom (A, B) composed of 16 tubes (C) with variable concentration of gadolinium (expressed as mg/ml, D), and filled with agar.

Figure 2: MRI acquisition and segmentation.
A set of four sequences were acquired, including a T1 weighted Turbo Field Echo (A), a T1-weighted Spin Echo (B), a Fluid Attenuated Inversion-Recovery (FLAIR, C), and a FLAIR with dedicated refocusing angle schemes (D). To extract mean signal intensity over six continuous slices, each tubes were segmented and labelled (E).
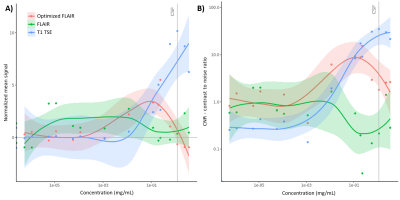
Figure 3: MR normalized signal and contrast-to noise ratio.
A) Normalized mean signal and standard error of T1 SE (blue line), FLAIR (green line), and optimized FLAIR (red line) for each concentration of gadolinium. For all concentrations, the contrast-to-noise ratio was computed (B), relative to the saline tube.
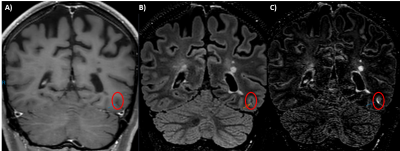
Figure 4: In-vivo preliminary evaluation.
A patient with Multiple Sclerosis (female, 31Y) underwent an MRI as part of her clinical follow-up, which includes a T1-weighted TSE (A), a FLAIR (B), and an optimized FLAIR (C). A linear leptomeningeal enhancement (red circle) was visible on the optimized FLAIR, while totally absent from the T1 TSE, and suspected on the conventional FLAIR.