3356
MRI System Phantom: assessing the MR visible thermometer, scanner geometric distortion corrections, and effect of fill conductivity1NIST, Boulder, CO, United States, 2Intermountain Neuroimaging Consortium, University of Colorado, Boulder, CO, United States, 3University of Colorado Anschutz, Radiological Sciences, Aurora, CO, United States, 4CaliberMRI, Inc., Boulder, CO, United States
Synopsis
Using the MRI system phantom we demonstrate the utility of the embedded MR-readable thermometer to measure and correct for temperature variations, demonstrate the use of the fiducial array to characterize scanner geometric distortions and the efficacy of software distortion corrections, and finally look at the effect of the electromagnetic properties of the phantom fill on quantitative measurements.
INTRODUCTION
NIST and the ISMRM ad-hoc Committee on Standards for Quantitative Magnetic Resonance, designed and commercialized a standard MRI system phantom1,2 to assess scanner performance, stability, inter-comparability and accuracy of quantitative relaxation time imaging3-5. Several important measurement protocols need to be improved and made more rigorous. The items addressed here are: 1) using the embedded liquid crystal (LC) MR-visible thermometer6 to automatically record phantom temperature; 2) ascertaining efficacy of geometric distortion corrections; and 3) determining the effects of the fill conductivity on the MR measurements.METHODS
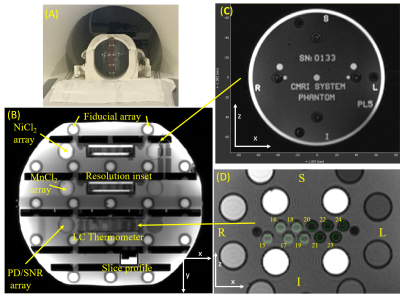
Two commercial MRI system phantoms were purchased7, along with reference solutions. The solution T1, T2 relaxation times were traceably calibrated at 3T over a temperature range from 16C to 26C. Geometric accuracy was calibrated using traceable CT. The system phantoms are included in the NIST/ NIBIB phantom lending library https://www.nist.gov/programs-projects/nistnibib-medical-imaging-phantom-lending-library and are available for check-out. The phantoms were characterized on a 3T scanner using a 32-channel head coil with the plates in the sagittal plane (Figure 1A), a 90° rotation of the phantom from its default orientation. All data were analyzed using an open-source package PhantomViewer (https://github.com/MRIStandards/PhantomViewer). The LC thermometer consists of 10 cylindrical cells with transition temperatures varying in 1C increments from 15C to 24C. Circular 4mm diameter regions of interest were used to collect signal data for thermometry. Geometric distortion analysis was done by fitting the fiducial array, consisting of 56 precisely-machined 10.0mm spheres on a 40.0mm grid (1). The phantoms were imaged with both a deionized water fill and a phosphate-buffered saline fill with 20C conductivities of < 10-4S/m and 0.43S/m, respectively. The saline conductivity was chosen to be comparable to the conductivity of human tissue.RESULTS
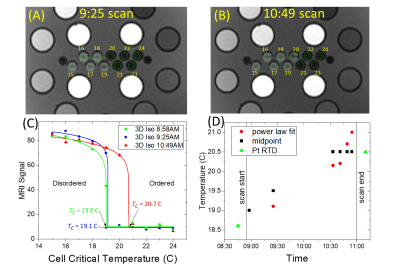
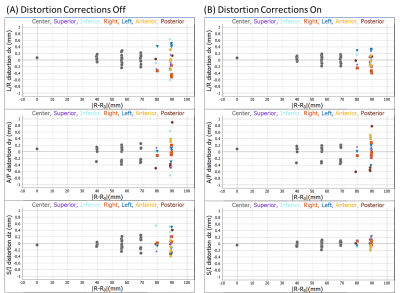
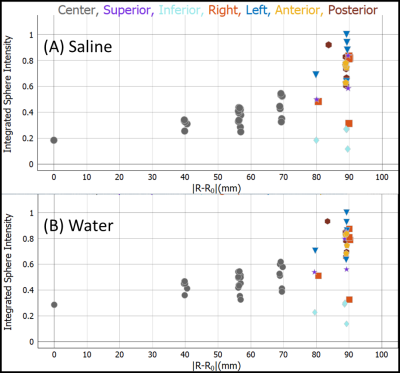
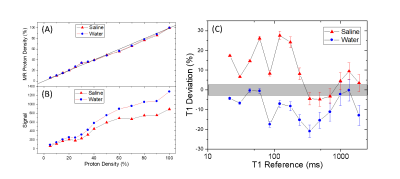
Figure 1 B,C,D show the system phantom configuration, the top plate with serial number, and the LC thermometer. The LC thermometer shows bright/dark cells where the LC critical temperatures are below/above the phantom temperature, respectively. The on-cells have a small ~ 1mm chemical shift artifact along the frequency-encode direction. Figure 2A,B show the LC thermometer at two points during the scanning session indicating the phantom is warming. Figure 2C shows the MR signal for each cell for three isotropic 3D scans taken at different times during the imaging session. The temperature can be determined by using a power law fit to the data, or by manually taking the midpoint between the last on-cell and the first off-cell critical temperature. These phantom temperatures are shown in Fig. 2D along with Pt-resistance thermometer measurements. Figure 3A,B show geometric distortion, defined as the difference of the apparent position of each fiducial sphere from the real position, obtained from a 3D GRE scan with and without software-based distortion corrections. The scans were done in the plane of the phantom plates, with the y-direction being perpendicular to the plates and scan plane. The software correction improves the in-plane distortion to < ±0.4mm and < ±0.2mm perpendicular and parallel to the scanner bore, respectively. The distortion correction applied here did not correct for the out of plane distortions. The change in fill causes a small shift in the image signal distribution across the phantom (Figure 4), with signal loss in the center of the phantom for the saline fill. A change in MR signal can also be observed for the proton density array (Figure 5) for the saline and water fills. The primary measurement, MR proton density, where the signal is normalized to the local background signal is not strongly affected. The biggest effect of the fill conductivity was observed in the variable flip angle (VFA) T1 protocol (Figure 5C). VFA is sensitive to nonuniformities in the RF transmit field which will be a function of the electromagnetic properties of the phantom fill. Other effects, including prescan shimming and RF power transmit/receive gains may have important effects which may lead to much greater variability in VFA.DISCUSSION
The MR-readable thermometer allows the temperature to be embedded in the MR data at scan time and provides a more reliable method of recording phantom temperature. The thermometer accuracy is ±0.5C, which is sufficient to push the thermal uncertainty for T1, T2 measurements on the NiCl2 array below 2%. The geometric distortion measurements are quick and useful ways to understand the intrinsic scanner accuracy due to nonuniform gradients as well as to verify that the software corrections are adequate. Figure 3B shows that current scanners can have geometric distortion less than 1 part in 103, hence calibration standards must exceed this accuracy. The effect of fill has a small effect on primary measurements but may have a considerable effect on less robust protocols such as T1 VFA. The effect and optimization of the electromagnetic properties of the phantom fill need to be further explored.CONCLUSION
Here we describe improvements and applications of the MRI system phantom including the incorporation of an MR-readable thermometer, simple evaluation of scanner geometric distortion and efficacy of distortion corrections, and the effect of varying the electromagnetic properties of the fill solution. While the MRI system phantom has limitations and considerable complexity, it provides necessary information and validation for many types of quantitative measurements.Acknowledgements
We thank all of the members of the ISMRM SQMR committee for input and guidance. We thank Nikki Rentz for long hours doing traceable NMR calibrations.References
1. Russek SE, Boss M, Jackson EF, Jennings DL, Evelhoch JL, Gunter JL, Sorensen AG. Characterization of NIST/ISMRM MRI System Phantom. Proc Intl Soc Mag Reson Med 20 2012:2456.
2. Keenan KE, Ainslie M, Barker AJ, Boss MA, Cecil KM, Charles C, Chenevert TL, Clarke L, Evelhoch JL, Finn P, Gembris D, Gunter JL, Hill DLG, Jack Jr. CR, Jackson EF, Liu G, Russek SE, Sharma SD, Steckner M, Stupic KF, Trzasko JD, Yuan C, Zheng J. Quantitative magnetic resonance imaging phantoms: A review and the need for a system phantom. Magnetic Resonance in Medicine 2018;79(1):48-61.
3. Bane O, Hectors SJ, Wagner M, Arlinghaus LL, Aryal MP, Cao Y, Chenevert TL, Fennessy F, Huang W, Hylton NM, Kalpathy-Cramer J, Keenan KE, Malyarenko DI, Mulkern RV, Newitt DC, Russek SE, Stupic KF, Tudorica A, Wilmes LJ, Yankeelov TE, Yen Y-F, Boss MA, Taouli B. Accuracy, repeatability, and interplatform reproducibility of T1 quantification methods used for DCE-MRI: Results from a multicenter phantom study. Magnetic Resonance in Medicine 2018;79(5):2564-2575.
4. Keenan KE, Gimbutas Z, Dienstfrey A, Stupic KF. Assessing effects of scanner upgrades for clinical studies. J Magn Reson Imaging 2019;50(6):1948-1954.
5. Jiang Y, Ma D, Keenan KE, Stupic KF, Gulani V, Griswold MA. Repeatability of magnetic resonance fingerprinting T1 and T2 estimates assessed using the ISMRM/NIST MRI system phantom. Magn Reson Med 2017;78(4):1452-1457.
6. Keenan KE, Stupic KF, Russek SE, Mirowski E. MRI-visible liquid crystal thermometer. Magn Reson Med 2020;84(3):1552-1563.
7. CaliberMRI. Web site. https://qmri.com/. 2020.
Figures