3354
Oil-in-water emulsions for tissue simulation in magnetic resonance imaging: Determination of MR-properties of emulsifiers1Section on Experimental Radiology, University of Tübingen, Tübingen, Germany, 2Institute for Diabetes Research and Metabolic Diseases of the Helmholtz Centre Munich at the University of Tübingen, Tübingen, Germany, 3Institute of Pharmaceutical Technology, University of Tübingen, Tübingen, Germany
Synopsis
The aim of this work was to develop stable and homogeneous oil-in-water emulsions for tissue simulation in MRI. For this purpose, three different emulsifiers (polysorbate 60, sodium dodecyl sulfate (SDS), and soy lecithin) were examined for their stabilizing ability. In addition, their potential impact on the MR-measurements was investigated. Sufficient stability can be achieved using both emulsifier, polysorbate and lecithin. Emulsions stabilized by SDS showed a visually lower stability. Due to its sufficient stabilizing ability, promising relaxometric properties (r1,lecithin=0,11wt%-1s-1, r2,lecithin =0,62wt%-1s-1), and no additional spectral resonances, lecithin is suggested as the preferred emulsifier for use in MRI.
Introduction
Tissue-like replications are used in many areas of MRI to optimize and calibrate imaging techniques.1-5 Emulsions are particularly suitable for simulation of in-vivo conditions, combining both, the properties of water and fat, and thus provide an excellent material for phantoms simulating tissue in MRI. However, a major problem with emulsions is their thermodynamic instability as they tend to separate in a water and an oil phase.6 A large number of emulsifiers such as polysorbates, sodium dodecyl sulfate or soy lecithin are used for stabilization. Those substances are usually not present in human tissues and their signals might lead to undesired effects.The overall purpose of this work was to develop stable and homogeneous oil-in-water emulsions for use in MRI. The potential impact of emulsifiers was investigated and analyzed. For a reliable simulation of tissue, it is essential that the emulsifiers used for stabilization have no or precisely calculable effects on the MR-measurements. Against this background, the suitability of various emulsifiers was examined in MRI and MRS experiments.
Methods
Data acquisition and analysis:Imaging and spectroscopy was performed on a 3.0T whole-body MR system (MAGNETOM Prismafit,SIEMENS Healthineers,Erlangen,Germany) using a standard 20-channel head coil. All data were carried out at room temperature of 22°C and analyzed offline using in-house-developed software (MATLAB,MathWorks,Natick,MA).
Preparation of oil-in-water emulsions:
Oil-in-water emulsions in volumes of 50ml were prepared using various types of emulsifiers. The composition of all samples was as follows: peanut oil: 20wt%; distilled water: 77wt%, and emulsifier: 3wt%. Polysorbate 60 (BASF,Ludwigshafen,Germany), sodium dodecyl sulfate (SDS,C.Roth,Karlsruhe,Germany) and soy lecithin (C.Roth,Karlsruhe,Germany) were used as emulsifier and trialed individually. The emulsifier concentration was chosen sufficiently high in order to prevent oil-droplets from coalescence during emulsification.
Emulsions were prepared by ultrasonication using UP200Ht (Hielscher Ultrasonics,Teltow,Germany). First, emulsifier solutions were prepared by dissolving the emulsifiers in distilled water. To increase solubility, the distilled water was heated to 30-40°C using a water bath. Then the amount of peanut oil was added to the solution while stirring gently. Finally, the mixtures were emulsified by sonication for 90s with an output of 70W. The prepared emulsions were kept in CELLSTAR polypropylene tubes (Greiner Bio-One,Frickenhausen,Germany) at room temperature.
Emulsion stability analysis:
Since T2-mapping enables monitoring of emulsion stability,7 the oil-in-water emulsions were scanned using a CPMG multiple spin-echo pulse sequence with a TR of 5000ms and 32 TEs ranging from 12ms to 384ms (increment:12ms). The selected slice was placed in a central plane through the samples and quantitative T2-maps were generated. Experiments were carried out 3h, 12h and 44h after preparation.
Spectroscopic characterization of the emulsifiers:
Additionally, a solution of each emulsifier (3wt%) in water was spectrally analyzed. A 1H-MR spectrum was acquired from each sample using a stimulated echo acquisition mode (STEAM) sequence with TR of 10000ms and TE of 10ms. The VOI was positioned in the center of each sample.
Relaxivity of aqueous solutions of emulsifiers:
Emulsifier solutions of varying concentrations (0wt%, 0.5wt%, 1wt%, 1.5wt%, 2wt%, 2.5wt%, 3wt%) have been prepared for polysorbate and lecithin. The solutions were kept in CELLSTAR tubes and fixed in a commercially available cylindrical phantom. Quantitative T1-maps in the coronal plane were acquired using inversion-recovery pulse sequence with seven different TIs ranging from 25ms to 8000ms. TR and TE were set to 10000ms and 9.9ms, respectively. Quantitative T2-maps were acquired using multiple spin-echo pulse sequence with a TR of 6000ms and 32 different TEs in the same slice. The initial and final TE were set to 50ms and 1600ms (increment:50ms), respectively.
Results
Emulsion stability analysis:Emulsions stabilized by SDS had a significantly lower stability compared to those stabilized by polysorbate and lecithin (fig.1). Emulsions prepared with polysorbate were the most stable. Creaming was hardly observed within the first 12h.
Spectroscopic characterization of the emulsifiers:
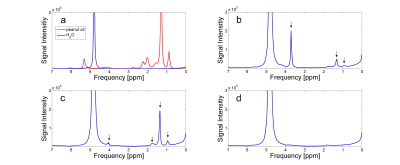
The results of the spectral analysis are shown in figure 2. Spectra of water and peanut oil were acquired as reference (fig.2a). Several peaks (indicated by arrows) could be observed for polysorbate and SDS (fig.2b-c). With the exception of a slight line broadening of the water signal, no further signals can be observed for lecithin (fig.2d).
Relaxivity of aqueous solutions of emulsifiers:
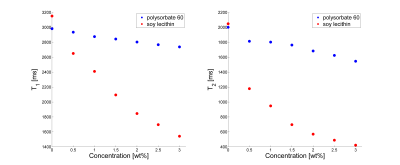
Relaxometry measurements revealed completely different influences of polysorbate and lecithin (fig.3). Polysorbate showed only minor effects on T1 and T2 of distilled water (relaxivity: r1,polysorbat=0.01wt%-1s-1 (R2 =0.99), r2,polysorbat=0.04wt%-1s-1 (R2=0.95)), whereas lecithin on the other hand showed a strong decrease in T1 and T2 with increasing concentration (relaxivity: r1,lecithin=0.11wt%-1s-1 (R2=0.99), r2,lecithin=0.62wt%-1s-1 (R2=0.99)).
Discussion and Conclusion
Sufficient stability can be achieved using both emulsifiers, polysorbate and lecithin. In contrast, SDS couldn’t be used as a suitable stabilizer with the preparation method chosen in this study. For SDS, creaming was observed immediately after preparation. The spectral analysis of the surfactants showed several additional resonances for polysorbate and SDS. Especially the resonances at 1.3ppm and 0.9ppm, which are usually seen in triglycerides, are considered to be critical with regard to fat quantification methods. In the case of lecithin, however, no conspicuous signals were detected. Due to this and the sufficient stabilizing ability, lecithin is suggested as the preferred emulsifier for use in MRI-experiments. In addition to its function as a stabilizer, lecithin can also be used to shorten relaxation times.Acknowledgements
No acknowledgement found.References
- Bernard CP, Liney GP, Manton DJ, Turnbull LW, Langton CM. Comparison of fat quantification methods: a phantom study at 3.0T. J. Magn. Reson. Imaging. 2008; 27(1):192-7.
- Fukuzawa K, Hayashi T, Takahashi J, Yoshihara C, Tano M, Kotoku J, Saitoh S. Evaluation of six-point modified dixon and magnetic resonance spectroscopy for fat quantification: a fat-water-iron phantom study. Radiol. Phys. Technol. 2017; 10(3):349-358.
- Poon CS, Szumowski J, Plewes DB, Ashby P, Henkelman RM. Fat/water quantitation and differential relaxation time measurement using chemical shift imaging technique. Magn. Reson. Imaging. 1989; 7(4):369-82.
- Schick F, Machann J, Brechtel K, Strempfer A, Klumpp B, Stein DT, Jacob S. MRI of muscular fat. Magn. Reson. Med. 2002; 47(4):720-7.
- Bush EC, Gifford A, Coolbaugh CL, Towse TF, Damon BM, Welch EB. Fat-Water Phantoms for Magnetic Resonance Imaging Validation: A Flexible and Scalable Protocol. J. Vis. Exp. 2018; (139):e57704.
- Kutz G, Daniels R, Trommer H. Emulsionen: Entwicklung, Herstellung, Prüfung. Aulendorf: ECV Editio-Cantor-Verlag, 2011.
- Onuki Y, Kida C, Funatani C, Hayashi Y, Takayama K. MRI as a promising tool for evaluation of the stability of cosmetic emulsions. Int. J. Cosmet. Sci. 2016; 38(3):272-8.
Figures

Figure 1: T2-maps of oil-in-water emulsions - acquired at different times after preparation. Assignment of the samples from the left to right: emulsion without emulsifier, stabilized by polysorbate, stabilized by lecithin, stabilized by SDS.