3345
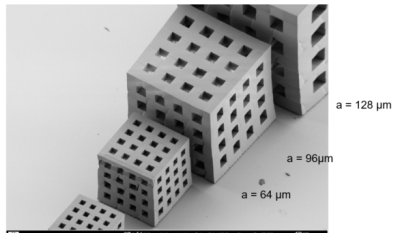
Cube set for the quantification of 3-dimensional spatial resolution in MR- micro imaging and microscopy (a = 128 - 4 µm) using 2-photon lithography1Center for Medical Physics and Biomedical Engineering High field MR-Center, Medical University of Vienna, Vienna, Austria, 2High-Field MR-Center (MRCE), Medical Universits of Vienna, Vienna, Austria, 3Karlsruhe Nano Micro Facility (KNMF), Karlsruhe Institute of Technology (KIT), Leopoldshafen-Eggenstein, Germany
Synopsis
Quality-control for systematic improvements in 3D-isotropic spatial resolution of the MR-imaging system up to the microscopic range becomes increasingly relevant not only for preclinical imaging but also for High-Field-MR human scanners. The design of a prototype phantom, comprising a set of 3D-cubes and an exemplary MR-microscopic evaluation is presented. The extraordinary challenges on accurate bar-width-to-cavity ratios for the cube range from periodicity a=128µm down to a=4µm are dealt with using 2-photon-lithography as manufacturing technology. The spatial resolution for 3D isotropic imaging can be checked qualitatively and quantitatively by interpolation of the Modulation-Transfer-Function demonstrated on a 3D-GRE sequence as an example.
Purpose/Introduction
The most important criterions for image quality in MR-imaging are related to the contrast-to-noise-ratio for the specific object and the spatial resolution of the MR imaging system. High spatial resolution based on voxel- and pixel-sizes (ps) in the microscopic range (ps < 100 x 100 µm2), is often claimed not only on preclinical animal and investigational vertical high-field MR-microscopy devices but also recently even on human MR-scanners1. However the spatial resolution is only ultimately limited by the voxel size. The actual achieved spatial resolution might be significantly worse and consequently the qualitative and quantitative check is recommended in several norms on quality control of human MR-scanners based on one dimensional periodic sets of plates or tubes2-4. The spatial resolution is dependent on several different technical and sample specific features, e.g. signal-to-noise ratio (SNR), bandwidth/pixel and gradient strength, line width (T2), susceptibility and chemical shift differences, B0-homogeneity and gradient switching behavior. Thus the improvement of hard- and software components of the MR-imaging system including hard- and software is to be proved at best for an adapted non-degradable resolution phantom presenting periodic structures in all of the three dimensions, not-only for the in-plane phase or frequency encoding direction5, but also the third slice-selective, phase or "super-resolution" encoding axis. Such phantoms do not exist yet for the spatial microscopy range with voxel sizes VS < 100 x 100 x 100 µm3.Subjects and methods
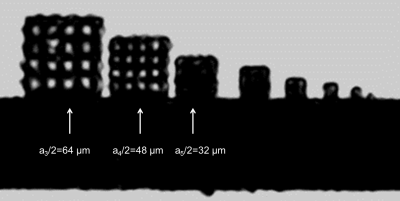
The design of the proposed reference resolution standard phantom for isotropic 3D- MR-micro-imaging and microscopy comprised a set of 3D- cavity containing cubes at identical bar-to-cavity ratio (duty cycle dc = 1) with min. 4 periods (periodic length: a, see fig. 1) for each spatial frequency ranging from a1 = 512 µm, down to a9 = 2 µm. Such 3D-structures would allow for checking spatial resolution from 2 to 500 line pairs (lp) per mm. The demands on miniaturization and spatial accuracy for the establishment of these reference objects at different characteristic structural widths of more than 6 orders of magnitude (106) were very challenging but could be (partly) fulfilled using 3D-Direct Laser writing (3D-DLW), based on a 2-photon absorption for localised polymerisation in a negative resist6. After 2 years of optimization we achieved a 3D-DLW manufacturing protocol for a cube set comprising 9 cubes (ai = 128, 98, 64, 48, 32, 24, 16, 8, and 4 µm), each offering 4.5 periods for the coverage of several modulations with excellent duty cycle (fig.1). This set of cubes was positioned in a small tube containing silicon oil for MR-visualization and checked for MR visibility. For demonstration of suitability for quality control on spatial resolution in MR-µ-imaging and MR-microscopy a prototype insert on a human High-field 7T scanner7 was used.Results
The 3D cube set allowed to quickly check the impact of several hardware, pulse sequence and MR-protocol parameters, e.g. bandwidth/pixel, frequency-/phase encoding and nominal slice thickness, on the spatial resolution in all of the 3 dimensions based on one MR-measurement data set. In most of the cases there is a significant difference between voxel size and actual spatial resolution. As an example the MR-microscopic image (sagittal view) resulting from the 3D-data set of a Cartesian frequency and phase encoding high-resolution 3D-gradient echo sequence is shown in fig. 3. Whilst the cube-grid with a/2 = 48 µm is resolved, the inner structure of the cube with ai = 32 µm is not detectable, though the nominal voxel size is less (31x31x30 µm3).Moreover the 3D- MR-microscopic data set on the 3D- resolution phantom also allows for the quantitative evaluation of the Modulation Transfer Function (MTF) in all of the 3 spatial dimensions for different spatial encodings. A quantitative criterion on the relative intensity modulation depth (Ir) might be used (e.g. Irres = 50% Irmax)8. In the case of the 3D-gradient echo (GRE) sequence this criterion, using a MTF fitting function, resulted in an effective spatial resolution of ares/2 = 45 µm (11,1 line-pairs/mm).
Discussion/Conclusion
Quantitative quality control on acutal achieved spatial resolution with difference to voxel size becomes increasingly important for the upcoming perspectives for MR-micro-imaging and microscopic resolution even on human scanners. With difference to previous investigations3,5 we propose a 3D-cube set concept covering the spatial range from ai = 512 µm to 1 µm. We could realize partly this design concept with a cube set ranging from ai = 128 µm down to ai = 4 µm using 3D-Direct Laser Writing (3D-DLW) based on the 2-photon initiated polymerization process. As an example the spatial resolution for a 3D-GRE sequence with nominal voxel VS ≈ (30 µm)3 is investigated, the difference between voxel size and spatial resolution is shown. The proposed investigational 3D phantom set might serve in future as basic concept for a standard on quantitative quality control referring to one of the most important image quality criterions in MRI: the spatial resolution.Acknowledgements
This project was supported by the KNMF-project nr. 2015-013-006488; A. Berg, 2-3-Dimensional μ-Structures for Magnetic Resonance Microscopy.References
1 Laistler, E., Dymerska, B., Sieg, J., Goluch, S., Frass‐Kriegl, R., Kuehne, A. and Moser, E. In vivo MRI of the human finger at 7 T. Magn. Reson. Med. 2018;79:588-592.
2 European Norm EN 61223-3-7 (IEC 62B/575/CDV) 2005.08.01. Evaluation and routine testing in medical imaging Departments; Part 3-7: Acceptance and constancy tests – Determination of essential image characteristics of magnetic resonance equipment.
3 American College of Radiology; MR accreditation program; Testing Instructions, small phantom guidance. 2018. https://www.acraccreditation.org/-/media/ACRAccreditation/Documents/MRI/SmallPhantomGuidance.pdf. Accessed December 11, 2020.
4 Fellner C, Muller W, Georgia J, Taubenreuther U, Fellner FA, Kalender WA; A high-resolution phantom for MRI; Magnetic Resonance Imaging 2001;19:899–904.
5 Berg A, Boerner M. Quantification of microscopic spatial resolution on MR-scanners using micro-phantoms manufactured with Deep-X-ray Lithography. Proc. Int. Soc. Mag. Reson. Med. 2020;28:4267.
6 Hengsbach S, Lantada AD. Rapid prototyping of multi-scale biomedical microdevices by combining additive manufacturing technologies. Biomed Microdevices. 2014;16(4):617-27.
7 Berg A, Potthast A, Starewicz P. MR-MICROSCOPY ON A HUMAN 7T-SCANNER; Proc. ISMRM 2010: progr nr. 1048.
8 Lerski RA, de Certaines JD. II Performance assessment and quality control in MRI by Eurospin test objects and protocols. Magnetic Resonance Imaging 1993;11(6):817-833.
Figures