3338
B1+ Homogenization in 3D Liver MRI at 7 Tesla Using Eight-Channel Parallel Transmission: Kt-points vs. Phase Shimming
Bobby Runderkamp1, Thomas Roos2, Wietske van der Zwaag2, Matthan Caan3, Gustav Strijkers3, and Aart Nederveen1
1Radiology and Nuclear Medicine, Amsterdam UMC, Amsterdam, Netherlands, 2Spinoza Center for Neuroimaging, Amsterdam, Netherlands, 3Department of Biomedical Engineering and Physics, Amsterdam UMC, Amsterdam, Netherlands
1Radiology and Nuclear Medicine, Amsterdam UMC, Amsterdam, Netherlands, 2Spinoza Center for Neuroimaging, Amsterdam, Netherlands, 3Department of Biomedical Engineering and Physics, Amsterdam UMC, Amsterdam, Netherlands
Synopsis
Abdominal MRI at ultra-high field benefits from increased SNR but is challenged by high B1+-inhomogeneity. We use eight-channel parallel transmission to homogenize B1+ signal in 7T liver MRI and compare CP-transmission against phase shimming and a kt-points pulse. Increased signal homogeneity was obtained with the kt-points pulse and phase shimming compared to CP-transmission for small volunteers. Combining the kt-points pulse with a compressed sensing reconstruction facilitated single breath-hold high-resolution liver MRI. With improved B1-mapping and kt-point optimization, kt-points is expected to also improve homogeneity in larger volunteers, and to outperform phase shimming in achieving homogeneous signal in 7T liver MRI.
Introduction
The signal gain facilitated by 7T MRI may be exploited to improve abdominal image quality. However at this high field strength, the B1+ wavelength becomes smaller than typical abdominal dimensions, which introduces B1+ inhomogeneities that cannot be corrected for with traditional circularly polarized (CP) transmission. The aim of this work was to use multi-channel parallel transmission to address B1+ inhomogeneities in the liver in 3D, comparing phase shimming with a kt-points pulse1. This was combined with SENSE and Compressed Sensing2 (CS) to enable high-resolution 7T liver MRI in a single breath-hold.Methods
Five healthy volunteers with a wide range in abdominal size were scanned with a 7T Philips Achieva scanner (Philips, Best, The Netherlands) using an 8-channel Tx/Rx fractionated dipole antenna body array3 (MRCoils, Netherlands, Figure 1b). For each volunteer, a 3D sub-Ernst angle GRE per transmit channel (single-channel-on) and a B1-map (DREAM4, all-channels-on, CP-transmission) were combined to calculate single-channel B1-maps. These B1-maps were noise-clipped to remove erroneous B1 values in areas with too low signal. Together with a 2nd order B0-shimmed 3D-GRE, these maps served as input for phase shimming and kt-points pulse calculation. Shimming volumes-of-interest (VOI) in the whole liver (volunteers 1,3,4 and 5) or part of the liver (volunteer 2) were manually drawn. For phase shimming, the 8 Tx-channel phase offsets were individually adapted to minimize the RF standard-deviation/(mean)2 in the VOI, using the MRCodeTool toolbox (MRCode, Netherlands). Kt-points pulses were calculated with five subpulses, max. blip strength = 33 mT/m, max. blip slew rate = 166 mT/m/ms and a maximum excitation k-space extent of 1/6 cm-1. Channel RF amplitudes and phases and the 3D excitation k-space trajectory were calculated using an interleaved greedy-local optimization algorithm5 in a home-built MRCodeTool extension6 (Figure 1d). To evaluate B1+-homogenization techniques, 3D-GRE scans were acquired using either CP-transmission, phase shimming, or kt-points. The 3D kt-points pulses were non-selective, requiring large FOVs to cover the entire transmission volume of the antennae. All scans were acquired in a single breath-hold. Details on the scan parameters are given in Figure 1a. To increase in-plane resolution while preserving scan-time, the kt-points acquisition was combined with SENSE and CS-acceleration (x3.65, volunteer 1). For CS, a random undersampling pattern was employed (Figure 1c) using the in-house developed PROUD software patch7,8. Reception coil sensitivity estimation from a fully sampled k-space center and image reconstruction were performed using BART9 and MRecon (Gyrotools, Zurich, Switzerland) in MATLAB. Regularization parameter optimization was performed heuristically. To assess image quality, signal intensity distributions in the 3D VOI for the CP-transmission, phase-shimmed and kt-points scans were evaluated.Results
Figures 2&3 respectively show two slices of the CP-transmission, phase-shimmed and kt-points scans, and figure 4 shows the signal intensity distributions in the VOIs for all volunteers. In the smallest volunteers, 1&2, phase shimming and kt-points show considerable increase in signal and homogeneity compared to CP-transmission. Kt-points shows slightly improved homogeneity over phase shimming in areas without noise-clipping, although small areas with signal dropout persist in noise-clipped B1 regions. A modest increase in signal is seen for volunteer 4&5 for phase shimming and volunteer 4 for kt-points. In volunteer 3, kt-points improved homogeneity; however, in the largest volunteer, 5, kt-points reduced image quality. Figure 5 shows two slices comparing the original kt-points scan with acceleration by SENSE and CS. The increased resolution improves depiction of fine structures at the cost of aliasing artifacts with SENSE. No such artifacts are present in CS-reconstructed images, at the expense of slightly decreased image quality in the lateral liver segment.Discussion
The results show that in volunteers with a small waist, kt-points and phase shimming can homogenize a considerable part of the liver. In areas where the B1-maps are measured correctly, kt-points performs superior over phase shimming. We think that the remaining small areas with signal dropouts might be improved by increasing the max. excitation k-space extent of the kt-points pulse and increasing the number of sub-pulses. Less improvement is seen deeper in the abdomen, where the B1-map has insufficient SNR and large areas were removed from the data using noise-clipping. As a result, for volunteers with a larger waist, the calculated excitation k-space trajectory was one in which all sub-pulses were emitted in the vicinity of a single k-space location. This results in a suboptimal pulse producing a gradient-like signal drop-off in the left-right axis. Possible improvements for this inadequate B1-map are increasing SNR by reducing resolution and increasing NSA, optimizing the noise clip factor or using Fourier-PE DREAM10. We also intend to make the kt-points sub-pulses slab-selective, allowing a smaller FOV to be scanned and thus a smaller voxel size. Finally, measuring homogeneity using flip angle distributions might be preferable over signal distributions.Conclusion
We were able to implement kt-points and phase shimming in 7T liver MRI using multi-channel parallel transmission for 3D B1+-homogenization. Increased signal and homogeneity were obtained with kt-points and phase shimming compared to CP-transmission for small volunteers. Combining kt-points with a compressed sensing reconstruction facilitated single breath-hold high-resolution liver MRI. With improved B1-mapping and algorithm optimization, kt-points is expected to outperform phase shimming in all body sizes and achieve a highly homogeneous excitation of the liver at 7T.Acknowledgements
No acknowledgement found.References
- Cloos MA, Boulant N, Luong M, Ferrand G, Giacomini E, Le Bihan D, Amadon A. kT-points: short three-dimensional tailored RF pulses for flip-angle homogenization over an extended volume. Magn Reson Med. 2012;67(1):72-80. doi: 10.1002/mrm.22978.
- Lustig M, Donoho D, Pauly JM. Sparse MRI: The Application of Compressed Sensing for Rapid MR Imaging. Magn Reson Med. 2007;58:1182-1195. doi: 10.1002/mrm.21391.
- Raaijmakers AJE, Italiaander M, Voogt IJ, Luijten PR, Hoogduin JM, Klomp DWJ, van den Berg CAT. The Fractionated Dipole Antenna: A New Antenna for Body Imaging at 7 Tesla. Magn Reson Med. 2016;75:1366-1374. doi: 10.1002/mrm.25596.
- Nehrke K, Versluis MJ, Webb A, Börnert P. Volumetric B1(+) mapping of the brain at 7T using DREAM. Magn Reson Med. 2014;71(1):246-56. doi: 10.1002/mrm.24667.
- Grissom WA, Khalighi M, Sacolick LI, Rutt BK, Vogel MW. Small-tip-angle spokes pulse design using interleaved greedy and local optimization methods. Magn Reson Med. 2012;68(5):1553-62. doi: 10.1002/mrm.24165.
- Roos T, Knapen T, van der Zwaag W. Reducing B1+ inhomogeneities in the brain at 7T: Pads or Parallel transmit? Proceedings of the 11th Annual Meeting ISMRM Benelux Chapter, 2019; Leiden, Netherlands
- Peper ES, Gottwald LM, Zhang Q, Coolen BF, van Ooij P, Strijkers GJ, Nederveen AJ. 30 times accelerated 4D flow MRI in the carotids using a Pseudo Spiral Cartesian acquisition and a Total Variation constrained Compressed Sensing reconstruction. Proceedings of the 27rd Annual Meeting ISMRM, 2018;Paris, France.
- Gottwald LM, Peper ES, Zhang Q, Coolen BF, Strijkers GJ, Planken RN, Nederveen AJ, van Ooij P. Pseudo Spiral Compressed Sensing for Aortic 4D flow MRI: a Comparison with k-t Principal Component Analysis. Proceedings of the 27rd Annual Meeting ISMRM, 2018;Paris, France.
- Uecker M. Berkeley Advanced Reconstruction Toolbox Target Audience: 2015;163(1991):25176. doi:10.1002/mrm.25176.
- Tse DHY, Poole MS, Magill AW, Felder J, Brenner D, Jon Shah N. Encoding methods for B1(+) mapping in parallel transmit systems at ultra high field. J Magn Reson. 2014;245:125-32. doi: 10.1016/j.jmr.2014.06.006.
Figures
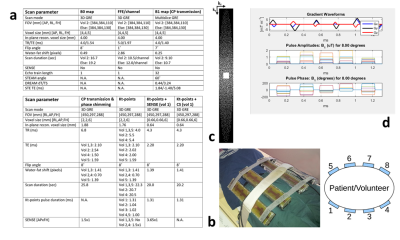
Figure 1: a)Parameters for the scans used for calculation of optimized phase offsets and
kt-points pulse, and the eventually B1+ homogenized scans. For kt-points scans, the echo
time was defined as the time between the echo and the middle of the kt-points pulse. b)The 8-channel fractionated dipole antenna body array used
for parallel transmission. In this study, the antennas were placed
asymmetrically towards the side of the body containing the liver. c)The
undersampling k-space pattern used in the CS scan. d)An exemplary kt-point pulse trajectory and pulse amplitudes and
phases.
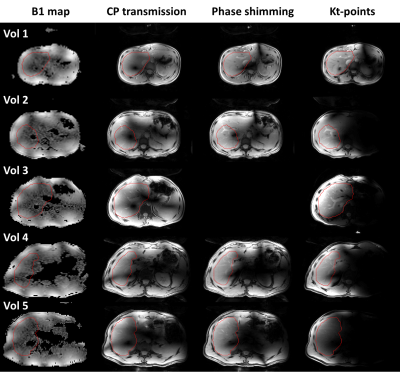
Figure 2: B1-maps
and magnitude images acquired with CP-transmission, phase shimming and kt-points,
at the level of the right portal vein, for all volunteers. The drawn shim VOI
in this slice is outlined in red. For volunteer 3, no phase shimmed image was
acquired. In the smallest volunteers, 1&2, phase shimming and kt-points
show considerable signal and homogeneity increase compared to CP-transmission. In the larger volunteers,
4&5, B1-maps appear more severely noise-clipped, resulting in lower to no
improvement using kt-points and phase shimming.
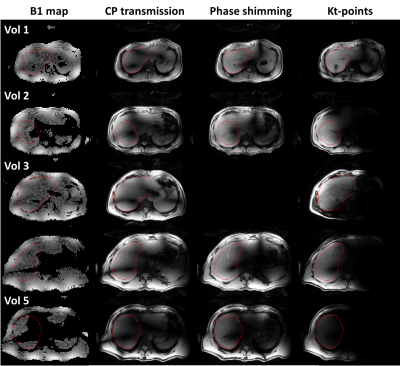
Figure 3:
B1-maps and
magnitude images acquired with CP-transmission, phase shimming and kt-points, 4
cm above the right portal vein, for all volunteers. The drawn shim VOI in this
slice is outlined in red. In the smallest volunteers, 1&2, phase shimming and kt-points show
considerable signal and homogeneity increase compared to CP-transmission. Volunteer 3 shows a modest
improvement with kt-points over CP-transmission. In the larger volunteers,
4&5, B1-maps appear more severely noise-clipped, resulting in lower to no
improvement using kt-points and phase shimming.
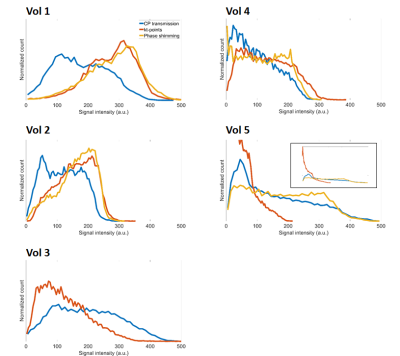
Figure 4:
Normalized
signal intensity distributions in the VOI of the 3D-GRE scans acquired using
CP-transmission (blue), kt-points (red) and phase shimming (yellow), for all
volunteers. In the smallest volunteers, 1&2,
phase shimming and kt-points show considerable signal and homogeneity increase
compared to CP-transmission. In
volunteer 3, improved homogeneity but decreased signal is observed using
kt-points compared to CP-transmission. In the larger volunteers, 4&5, lower
to no improvement using kt-points and phase shimming is observed.
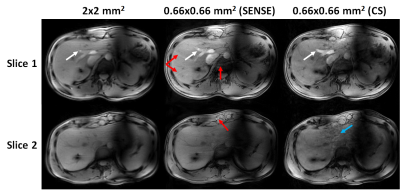
Figure 5: Magnitude
images (volunteer 1) acquired using kt-points with in-plane resolution of 2x2
mm2 (left), 0.66x0.66 mm2 with SENSE-acceleration
(middle) and 0.66x0.66 mm2 with CS-acceleration (right). Two slices
are shown at the same locations as figures 1&2. The increased resolution
improves depiction of fine structures (white arrows) at the cost of aliasing
artifacts with SENSE (red arrows). No such artifacts are present in CS
reconstructed images, at the expense of slightly decreased image quality in the
lateral liver segment (blue arrow).