3310
Prospective Accelerated Cartesian 3D-T1rho Mapping of Knee Joint using Data-Driven Optimized Sampling Patterns and Compressed Sensing1Radiology, NYU Langone Health, New York, NY, United States, 2Siemens Healthineers, New York, NY, United States
Synopsis
We modified the Cartesian 3D-fast spoiled gradient-echo sequence with T1rho magnetization preparation for prospective acceleration of knee-joint mapping using optimized sampling patterns (SPs) and compressed sensing (CS) reconstructions. In this sequence, after each T1rho preparation module, several k-space lines are captured, partially filling the 3D k-space. However, the ordering of the k-space filling is very important to maintain consistent T1rho contrast and to obtain stable quantitative mapping. This is even more challenging when arbitrary SPs are used for accelerated MRI. We investigate different k-space ordering schemes considering optimized SPs and Poisson disk SPs in prospective 3D-T1rho acquisition with CS reconstructions.
Introduction:
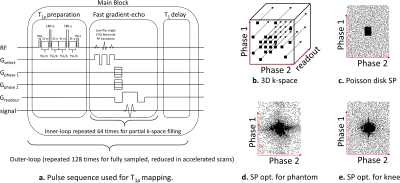
Previous retrospective studies demonstrated that joint parallel MRI and Compressed Sensing (CS) is effective for accelerated T1rho mapping of knee-joint [1]–[3]. However, no prospective accelerated methods have been investigated for knee joint applications yet. Also, data-driven optimized sampling patterns (SPs), obtained using machine learning methods such as [4], [5], have shown an increase in accelerated image quality. To make use of these SPs, pulse sequences for T1rho mapping of knee-joint have to be modified to use prospective acceleration with optimized SPs.The 3D-T1rho pulse sequence used in [1]–[3], [6], [7] was modified to accept any externally-defined SP. This implemented 3D-T1rho sequence is based on fast gradient-echo [8] and collects several 3D k-space lines after each T1rho-magnetization preparation [9], partially filling the k-space (see Fig. 1a) at each block. The main block of the sequence is repeated until enough data is captured.
Final T1rho contrast is affected by the order of k-space filling, particularly when the center is captured. Following [10]–[12], we investigated sequences that capture k-space center first, just after the preparation module. The reasoning is that T1rho magnetization reduces over time, affecting the later echoes in the block.
Methods:
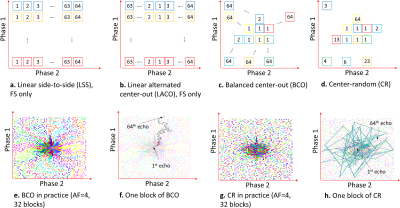
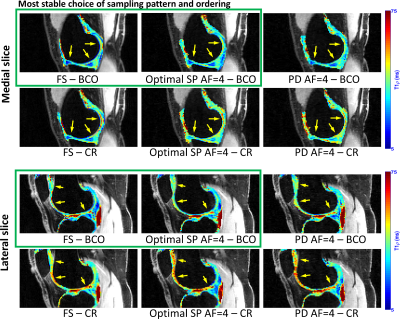
The imaging sequence (see Figure 1a) contains T1rho-magnetization preparation pulses [spin-lock frequency=500Hz, variable spin-lock time (TSL)], followed by a low flip-angle fast gradient-echo sequence (TurboFLASH, Siemens) [flip-angle(FA)=8deg]. The T1rho preparation is applied to the entire volume. The following TurboFLASH sequence collects 64 k-space readouts (256 samples each). Each k-space readout has a unique 2D phase-encoding position (defined as Phase1×Phase2, Figure 1b), perpendicular to the readout direction. This main block has a TR=1.3sec and it is repeated 128 times for fully-sampled 3D k-space acquisitions of size 256x128x64 (readout×Phase1×Phase2) with a fixed TSL, taking 2min46sec. Standard machine-defined ordering collects all 64 Phase2 positions in one fixed Phase1 position per block.Our modified T1rho sequence has more flexibility regarding the selection of the 2D phase-encoding positions (defined by the SP). In this study, we have investigated two SPs: Poisson disk and optimized SP [obtained using bias-accelerated subset selection (BASS)[5]], as shown in Fig. 1c-1d. However, the best order of data collection is unknown. Phase-encoding points need to be grouped into blocks of 64 and ordered. There is no significant reduction of time if less than 256 samples per readout or 64 points per block are captured. The order of the 64 readouts in each block is important because the T1rho contrast is gradually reduced over time, after T1rho preparation. This way, phase-encoding positions related to the central area of the k-space have to be acquired right after each preparation module. We investigated two customized orderings schemes: balanced center-out (BCO) and center-random (CR), and two standard machine-provided ordering: linear alternated center-out (LACO) and linear side-to-side (LSS); see Figure 2a-b.
In the BCO scheme, the phase-encoding positions are ordered in the following manner: the centermost k-space positions are assigned one to each block. After that, one block at a time receives one unselected position that is simultaneously closest to the center and closest to the previously selected point of the block. The CR scheme selects first the centermost point for each block (same way as BCO), but after it assigns the points at random. Figures 2c-f illustrate the ordering schemes.
Experimental Details:
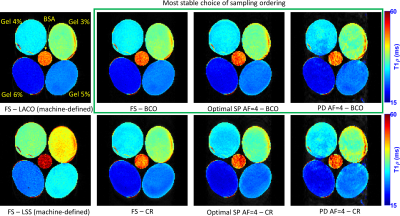
The SPs used was Poisson disk [1], [6] and the optimized SPs [obtained using [5] with 65 fully-sampled images]. Note the optimized SP for phantoms (Figure 1d) is different from the SP optimized for the human knee (Figure 1e). CS reconstruction was performed with MFISTA-VA [13] with spatial finite differences. T1rho mapping was obtained using a complex-valued fitting with mono-exponential models using non-linear least squares [3].We first evaluated the T1rho sequence on model phantoms [3%,4%,5% and 6% agar gel and 15% cross-linked bovine serum albumin (BSA)], to measure the stability of the T1rho maps for various ordering schemes [fully-sampled (FS) and accelerated 4 times (AF=4)]. Later, we also evaluated knee joint imaging on three healthy volunteers (n=3, mean age=26.6±1.5) using TSL=4ms,7ms,13ms,25ms,45ms.
Results and Discussion:
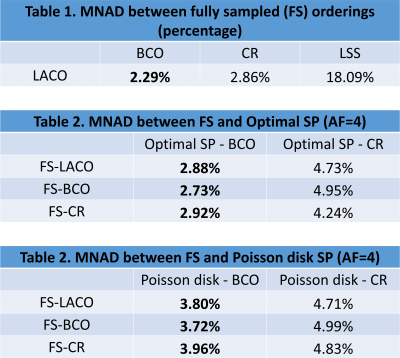
Figure 3 shows tables with the median of the normalized absolute differences (MNAD) (details in (3)) in model phantoms between the maps obtained with each ordering for FS and accelerated methods. The importance of the ordering, especially capturing the k-space center first, is illustrated by the large MNAD obtained by fully-sampled LSS (18.09%, in Table 1). The BCO ordering with optimized SP obtained the best (lower) results (2.73%~2.92%, in Table 2), superior to the second best, BCO with Poisson disk SP (3.72%~3.96%, in Table 3). CR ordering was less effective (4.24%~4.99%, in Tables 2 and 3). Figure 4 illustrates quantitative T1rho maps for model phantoms and Figure 5 for knee-joint mapping. FS acquisitions take 2min46sec to collect all 128 blocks per volume, totalizing 13min53sec for all 5 TSLs, while AF=4 schemes take 41.6sec to collect 32 blocks per volume (each block takes 1.3sec), totalizing 3min28sec for all 5 TSLs.Conclusion:
The proposed balanced center-out ordering has demonstrated least error and robusteness for T1rho mapping in this prospective study with accelerated acquisitions, especially with optimized SP. The modified T1rho sequence was able to handle different SPs well, including optimized SPs.Acknowledgements
This work was supported in part by NIH grants R21 AR075259, R01 AR076328, R01 AR067156, R01 AR070297, and R01 AR068966,and was performed under the rubric of the Center for Advanced Imaging Innovation and Research (CAI2R, www.cai2r.net) an NIBIB Biomedical Technology Resource Center (NIH P41 EB017183).References
[1] M. V. W. Zibetti, A. Sharafi, R. Otazo, and R. R. Regatte, “Accelerating 3D-T1ρ mapping of cartilage using compressed sensing with different sparse and low rank models,” Magn. Reson. Med., vol. 80, no. 4, pp. 1475–1491, Oct. 2018.
[2] M. V. W. Zibetti, R. Baboli, G. Chang, R. Otazo, and R. R. Regatte, “Rapid compositional mapping of knee cartilage with compressed sensing MRI,” J. Magn. Reson. Imaging, vol. 48, no. 5, pp. 1185–1198, Nov. 2018.
[3] M. V. W. Zibetti, A. Sharafi, R. Otazo, and R. R. Regatte, “Accelerated mono‐ and biexponential 3D‐T1ρ relaxation mapping of knee cartilage using golden angle radial acquisitions and compressed sensing,” Magn. Reson. Med., vol. 83, no. 4, pp. 1291–1309, Apr. 2020.
[4] M. V. W. Zibetti, G. T. Herman, and R. R. Regatte, “Data-driven design of the sampling pattern for compressed sensing and low rank reconstructions on parallel MRI of human knee joint,” in ISMRM Workshop on Data Sampling and Image Reconstruction, 2020.
[5] M. V. W. Zibetti, G. T. Herman, and R. R. Regatte, “Fast Data-Driven Learning of MRI Sampling Pattern for Large Scale Problems,” arXiv Prepr., pp. 1–18, Nov. 2020.
[6] M. V. W. Zibetti, A. Sharafi, R. Otazo, and R. R. Regatte, “Compressed sensing acceleration of biexponential 3D-T<inf>1ρ</inf> relaxation mapping of knee cartilage,” Magn. Reson. Med., vol. 81, no. 2, 2019.
[7] M. V. W. Zibetti, P. M. Johnson, A. Sharafi, K. Hammernik, F. Knoll, and R. R. Regatte, “Rapid mono and biexponential 3D-T1ρ mapping of knee cartilage using variational networks,” Sci. Rep., vol. 10, no. 1, p. 19144, Dec. 2020.
[8] Z. P. Liang and P. C. Lauterbur, Principles of magnetic resonance imaging: a signal processing perspective. IEEE Press, 2000.
[9] M. Gram, M. Seethaler, P. M. Jakob, and P. Nordbeck, “Balanced spin-lock preparation for B1 -insensitive and B0-insensitive quantification of the rotating frame relaxation time T1ρ,” no. May, pp. 1–10, 2020.
[10] D. Brenner, R. Stirnberg, E. D. Pracht, and T. Stöcker, “Two-dimensional accelerated MP-RAGE imaging with flexible linear reordering,” Magn. Reson. Mater. Physics, Biol. Med., vol. 27, no. 5, pp. 455–462, Oct. 2014.
[11] F. Lugauer, J. Wetzl, C. Forman, M. Schneider, B. Kiefer, J. Hornegger, D. Nickel, and A. Maier, “Single-breath-hold abdominal T1 mapping using 3D Cartesian Look-Locker with spatiotemporal sparsity constraints,” Magn. Reson. Mater. Physics, Biol. Med., vol. 31, no. 3, pp. 399–414, 2018.
[12] G. M. Beck, J. De Becker, A. C. Jones, M. von Falkenhausen, W. A. Willinek, and J. Gieseke, “Contrast-enhanced timing robust acquisition order with a preparation of the longitudinal signal component (CENTRA plus) for 3D contrast-enhanced abdominal imaging,” J. Magn. Reson. Imaging, vol. 27, no. 6, pp. 1461–1467, May 2008.
[13] M. V. W. Zibetti, E. S. Helou, R. R. Regatte, and G. T. Herman, “Monotone FISTA with variable acceleration for compressed sensing magnetic resonance imaging,” IEEE Trans. Comput. Imaging, vol. 5, no. 1, pp. 109–119, Mar. 2019.
Figures