3278
MRI-ASL Perfusion patterns may predict deep brain stimulation outcome in de novo Parkinson’s Disease1Center for biomedical imaging research, Tsinghua University, Beijing, China, 2Department of Neurosurgery, Tsinghua University Yuquan Hospital, Beijing, China, 3School of Computer Science and Technology, Beijing Institute of Technology, Beijing, China
Synopsis
Deep brain stimulation(DBS) is routine treatment in Parkinson’s disease(PD) but its outcome varies among patients. It’s of benefit to investigate predictive value of pre-operative MR perfusion patterns in DBS outcome. In this study, regional cerebral blood flow(CBF) were measured by ASL both medication “on” and “off” before DBS surgery. Machine learning models were applied to select features and to predict the DBS outcome. Pre-operative MR perfusion patterns shows valuable to predict the DBS outcome in de novo PD patients. The Ridge regression performed best with good correlation(r=0.87,p<0.001) and good agreement with the measured DBS outcome at error level of 9.0%.
Introduction
Deep brain stimulation(DBS) has shown better post-surgical improvements in movement and quality of life comparing to pharmacological dopamine therapies in PD1. However, as an invasive and costly therapy, DBS shows different outcomes among PD patients2. The pre-operative evaluation of individual DBS outcomes will not only help the doctors but the patients to make a thorough treatment consideration. PD-specific metabolism and perfusion patterns measured by imaging techniques have been established as a biomarker to reveal the severity, progression, and treatment responses3. The potential value of cerebral perfusion to predict DBS outcome in PD patients still remains unclear. By utilizing non-invasive ASL MRI, we aim to train a machine learning model to predict the outcome (percentage improvement of Unified Parkinson Disease Rating Scale, UPDRS) of de novo PD patients from the perfusion patterns.Methods
Demographic info and DBS surgery: 48 de novo PD patients (age: 60.0 ± 9.9 years old, H&Y scale 3.7 ±0.52) underwent DBS surgery was recruited. In pre-operative assessment, MRI scanning, and Movement Disorder Society (MDS)-UPDRS 2&3 were conducted to evaluate the cerebral perfusion, quality of life, and motor ability of the patients, respectively. Each patient was scanned and evaluated both medication “on” and “off” status with a time interval of more than 50 min. In post-operative assessment, UPDRS 2&3 were examined before discharge. All DBS surgeries were conducted by well-experienced surgeons and the electrode pins were well-positioned within the bilateral subthalamic nucleus.Imaging Protocol: All scans were obtained on a 3T MR scanner (Achieva, Philips Medical System, Best, The Netherlands) with a 32-channel head coil. pCASL and 3D T1w scans were examined successively with parameters: 3D T1w turbo field echo (TFE): sagittal acquisition with 256(FH)×256(AP)×160(RL) mm3 FOV; 1×1×2 mm3 resolution; TE/TR/Flip angle=3.6 ms/7.8 ms/8°. PCASL, axial acquisition with 240(AP)×240(RL)×132(FH) mm3; 3×3×6 mm3 resolution, TR/TE = 4013/12 ms, SENSE acceleration = 3, label duration = 1650 ms, post labeling delay (PLD) = 1575 ms. T1w images were acquired only for medication “off” while pCASL images were acquired for both medication “on” and “off” status with the same parameters.
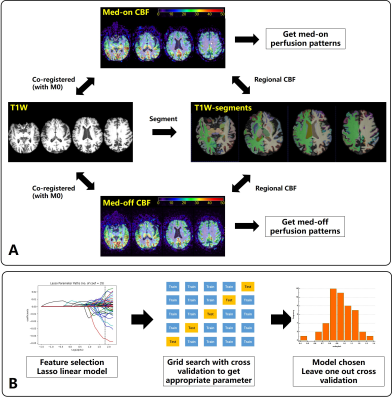
Image analysis (Figure 1 A): A deep learning-based neuroimaging process pipeline, Fastsurfer4, was utilized to segment the brain structures in 95 classes (33 for subcortical and 62 for cortical structures) on T1w anatomical images. CBF maps were calculated from raw pCASL data by Philips workstation. Both medication “on” and “off” pCASL M0 images were rigidly co-registered to the corresponding T1w native space via ANTs software5. The same transformation was applied to ASL-CBF map to calculate the regional CBF value. All segmentation and registration procedure were inspected by an experienced radiologist to avoid errors induced by image processing.
Construction and evaluation of predictive model (Figure 1 B): CBF mean value and stand deviation were calculated for each brain structure as the perfusion features of the PD patients. The predictive model took perfusion features combined with clinical information (disease course, age, sex, Hoehn and Yahr scale of PD) as input and took the medication ”off” UPDRS improvement after DBS therapy as output. To select the most important characteristics, feature selection was performed by Lasso linear model with self-chosen hyperparameters by cross-validation to minimize Mean squared error(MSE). Four commonly used machine learning models (Ordinary least square, OLS; Ridge regression; Decision tree regression, DTR; Support vector regression, SVR) was included with a 5-fold cross-validation grid search to choose the optimized model parameters. 40 of 48 patients’ data were constructed as the training dataset to find the appropriate model parameter. By comparing the mean absolute error(MAE), MSE, and R2 score, the model with the best performance was chosen and was assessed by leave-one-out cross-validation (LOOCV). Correlation, Bland-Altman plot, and paired t-test were utilized to compare the predictive and measured DBS improvements.
Results
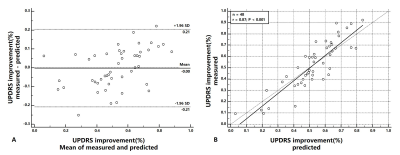
As shown in Table 1., 35 features were chosen from Lasso linear model. Taken selected features as model input, cross-validation was conducted on training dataset to get the best model parameter. All four models with appropriate model parameters were compared in Table 2. With the best performance, ridge regression was considered to be the predictive model. LOOCV showed the chosen model having a good correlation (r=0.81, p<0.001) and the Bland-Altman analysis with paired t-test (p=0.94) showed good agreement between predicted and measured DBS improvement with a mean predictive error of 9.0% UPDRS improvement(Figure 2.).Discussion and Conclusion
In this study, we constructed a machine learning model to predict the DBS outcome by pre-surgical perfusion. Lasso regression was utilized to select important features and cross-validation on train dataset was applied to choose the best model with appropriate parameters. Preliminarily, we proved brain perfusion patterns in both medication “on” and “off” status can be some predictors to guide the DBS treatment of PD. More fundamental research and a larger dataset may validate and extend this work in the future.Acknowledgements
No acknowledgement found.References
1. Schuepbach WM, Rau J, Knudsen K, Volkmann J, et al. Neurostimulation for Parkinson's Disease with Early Motor Complications. New England Journal of Medicine 2013; 368:610-622.
2. Cury RG, Galhardoni R, Fonoff ET, et al. Effects of deep brain stimulation on pain and other nonmotor symptoms in Parkinson disease. Neurology 2014; 83:1403–1409.
3. Rane S, Koh N, Oakley J, et al. Arterial spin labeling detects perfusion patterns related to motor symptoms in Parkinson's disease. Parkinsonism & Related Disorders 2020;76:21-28.
4. Henschel L, Conjeti S, Estrada S, et al. FastSurfer - A fast and accurate deep learning based neuroimaging pipeline. NeuroImage 2020; 219,117012.
5. https://github.com/ANTsX/ANTs
Figures