3277
Prediction of iron rim lesions in multiple sclerosis using convolutional neural networks and multi-contrast 7T MRI data1Department of Medical Engineering, Carinthia University of Applied Sciences, Klagenfurt, Austria, 2Department of Neurology, Medical University of Vienna, Vienna, Austria, 3School of Information Technology and Electrical Engineering, The University of Queensland, Brisbane, Australia, 4Department of Biomedical Imaging and Image-guided Therapy, High Field Magnetic Resonance Centre, Vienna, Austria
Synopsis
In multiple sclerosis (MS) the presence of paramagnetic iron rim lesions has been shown to be indicative for progression with a more severe disease course. Our goal was to develop a pipeline based on neural networks to automatically detect, segment and classify lesions as either non-iron or iron loaded using multi-contrast 7T MRI data. A patch-based approach with two modified u-net architectures was used for segmentation and classification. Automatic, high quality lesion segmentation and their classification based on the presence or absence of iron-rims is enabled using convolutional neural networks.
Introduction
Multiple Sclerosis (MS) is a chronic inflammatory demyelinating disease of the central nervous system, affecting at least 2 million people worldwide.1 The presence of iron containing microglia and macrophages at the edge of MS lesions has been shown to play an important role in disease progression and are associated with a more severe disease course.2 Convolutional neural networks (CNNs) have the potential to learn unique image features from high-dimensional input data. CNNs have proven to be robust and accurate methods in the field of image segmentation and could be implemented as a second rater for decision support.3,4 In this study, our goal was to build and evaluate a CNN for automatically segmenting and detecting iron rim lesions in MS using multi-contrast 7T MRI data.Material and Methods
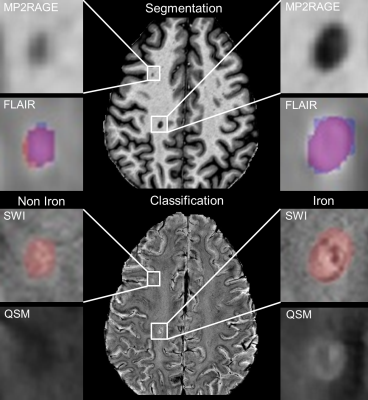
T1w, FLAIR, SWI and QSM data were acquired from 22 MS patients using a 7 T whole-body MRI system (Magnetom Terra, Siemens Healthineers, Erlangen, Germany) equipped with a 32-channel head coil (Nova Medical, Wilmington, USA). All images were resampled to the same resolution of 0.16 x 0.16 x 0.4 mm. An image segmentation and classification network was developed based on the common U-Net architecture.5 Similar to the standard U-Net, our two networks consist of a contracting and an expanding part. Skip connections between both parts copy feature maps from the downsampling branches and concatenate them with the upsampling branch to compensate for the spatial information loss during downsampling. For the lesion segmentation task, a multi-view network (axial, coronal and sagittal orientation) with T1 and FLAIR data as input was used. Individual segmentation probability maps (axial, coronal and sagittal) were afterwards fused by pixel-wise averaging and thresholded at 0.5. For iron classification, lesion feature-extraction was done with axial T1, SWI and QSM images multiplied by the binary lesion mask. Subsequently, different combinations of input data (QSM+SWI, QSM+SWI+T1, QSM+T1 and SWI+T1) were tested to identify which modalities contain the necessary information. Binary lesion masks and non-iron/iron labels were created by an MS expert with 10 years’ 7T MRI experience. For training, 188x188 pixel patches were extracted. Data augmentation including rotation, scaling, shifting and flipping was carried out to increase the amount of training data and to ovoid overfitting of the network, overall 15,000 patches were used. Prior to the segmentation process, all images were preprocessed including skull stripping, N4-bias correction6, intensity normalization (0-1) and intra-subject image registration. The neural networks were trained on 80% and validated on 20% of all image patches using the Adam optimizer with a learning rate of 0.0001. To test the network, six new MS patient data sets were used, which were not applied for training and validation. Lesion-wise true positive rate (LTPR) and lesion-wise false positive rate (LFPR) was calculated for the segmentation as well as for the classification process. For iron classification, receiver operating curves (ROC) analyses were performed.Results
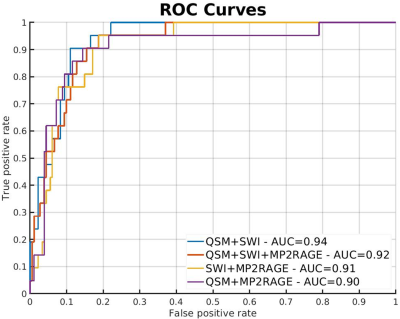
Fig. 1 illustrates the segmentation and classification results of the neural network using 7T MRI scans. Fig. 1 top row, a comparison between manual (blue) and CNN lesion segmentations (red) is shown. Quantitatively, we observed a mean LTPR of 90% and a mean LFPR of 23% in the six test MS patients. For the iron classification task, ROC curves are shown in Fig. 2. The highest area under the curve (AUC) value was achieved with the input combination of QSM and SWI (AUC=0.94), while lowest AUC values were observed from both combinations T1 with QSM or SWI. An example for correctly, iron based, classified lesions is shown in Fig 1. A sensitivity of 90% and a specificity of 89% was reached for the combination of QSM and SWI data, calculated at the optimal threshold that maximizes the Youdens index (sensitivity + specificity - 1).Discussion
We developed a pipeline based on two CNNs for detecting and classifying MS lesions that supports multiple high-resolution input contrast channels. First, our segmentation network delivers high quality lesion segmentations using a multi-view approach. Second, we can automatically classify MS lesions based on the presence or absence of an iron-rim. The use of this pipeline can therefore facilitate robust and automatic detection of lesions and thus help to better understand and characterize MS lesions. The ROC analysis revealed that QSM + SWI are best suited to predict the iron characteristics of the lesions.Conclusion
In conclusion, our pipeline is capable of automatically detecting, segmenting and classifying MS lesions with multi-contrast, high spatial resolution 7T data.Acknowledgements
No acknowledgement found.References
1. Tullman MJ. Overview of the epidemiology, diagnosis, and disease progression associated with multiple sclerosis. Am J Manag Care. 2013;19(2 Suppl):S15-20.
2. Absinta M, Sati P, Masuzzo F,
et al. Association of Chronic Active Multiple Sclerosis Lesions With
Disability In Vivo. JAMA Neurol.
2019;76(12):1474-1483. doi:10.1001/jamaneurol.2019.2399
3. Brugnara G, Isensee F,
Neuberger U, et al. Automated volumetric assessment with artificial neural
networks might enable a more accurate assessment of disease burden in patients
with multiple sclerosis. Eur Radiol. 2020;30(4):2356-2364.
doi:10.1007/s00330-019-06593-y
4. Eitel F, Soehler E,
Bellmann-Strobl J, et al. Uncovering convolutional neural
network decisions for diagnosing multiple sclerosis on conventional MRI using
layer-wise relevance propagation. Neuroimage Clin. 2019;24:102003.
doi:10.1016/j.nicl.2019.102003
5. Ronneberger O, Fischer P, Brox T. U-Net: Convolutional Networks for Biomedical Image Segmentation. In: Navab N, Hornegger J, Wells WM, Frangi AF, eds. Medical Image Computing and Computer-Assisted Intervention – MICCAI 2015. Springer International Publishing; 2015:234-241.
6. Tustison NJ, Avants BB, Cook
PA, et al. N4ITK: improved N3 bias correction. IEEE Trans Med Imaging. 2010;29(6):1310-1320.
doi:10.1109/TMI.2010.2046908
Figures