3255
Deep learning based high resolution IVIM parameter mapping in lacunar infarction patients1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Human Phenome Institute, Fudan University, Shanghai, China, 3Philips Healthcare, Shanghai, China
Synopsis
This study aimed to propose a reliable deep learning based high resolution intravoxel incoherent motion (IVIM) parameter mapping for the assessment of lacunar infarction patients.
INTRODUCTION
Simultaneous measurement of cerebral blood flow (CBF) and apparent diffusion constant (ADC) could provide important diagnostic and prognostic information for patients1. Recent studies2-3 have indicated that the directional sensitivity of the intravoxel incoherent motion (IVIM) signal to flow could characterize the direction-dependent flow network in cerebral vasculature and assess ischemic infarction and perfusion in patients with acute ischemic stroke. Generally, IVIM can measure both water molecule diffusivity and perfusion information with a variable range of b values using a double-exponential model. The IVIM signal is often averaged over a region of interest (ROI) to improve SNR and stability to avoid overfitting problems. However, the sensitivity of ROI-based approaches depends on the visual inspection of the ROIs. Recent researches4-5 showed that diffusion weighted images with higher resolution could provide the higher sensitivity of detecting ischemic lesions in TIA patients.This work aimed to evaluate the performance of a deep learning-based high resolution multi-shot IVIM parameter mapping method in assessment of lacunar infarction patients.
METHODS
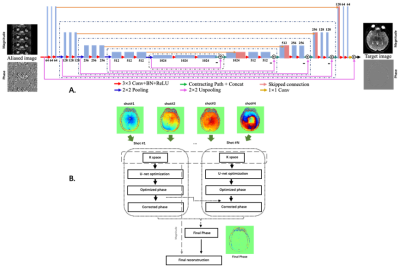
MR Imaging: All MRI experiments were performed on a Philips 3.0 T clinical scanner (Philips Healthcare, Best, the Netherlands). Brain images were acquired from 17 patients (mean age, 58.4 years; range, 48~71 years; 9F/8M) with lacunar infarction using a 32-channel phase-array head coil. All DWI data were acquired with 4-shot interleaved multi-shot (MSH) EPI DWI sequence from 20 slices, covering the whole brain with slice gap of 6 mm. Other parameters were shown as following: FOV=228×252 mm2, acquisition matrix=228×252, special resolution=1×1×5 mm3, TR/TE=2800/65 ms, scan time ≈400 s, b values = 0, 20, 40, 60, 80, 100, 120, 200, 400, 600, 800 and 1000 s/mm2 without additional average. Single-shot (SSH) EPI DWI were obtained with the same ETL and diffusion weighting protocols.Deep learning reconstruction: We trained the network on SSH-EPI data and applied the model to MSH-EPI. The aliased images were generated by down-sampling the full k-space with the same under-sampling factor and trajectory as MSH-EPI. Dual-channel U-net including multiple convolutional layers is used for phase estimation of MSH-EPI. The schematic diagram of deep learning based MUSE6 is shown in Fig. 1. Similar to conventional MUSE, images were reconstructed by incorporating the corrected phase into the final reconstruction. Instead of using SENSE for phase estimation, our method is performed by U-net with k-space data from different shots of DWI.
By incorporating U-net into conventional MSH-EPI reconstruction method7, the efficiency and performances of MSH-EPI diffusion weighted images could be improved. Meanwhile, the reconstruction of MSH-EPI using conventional model-base method and SSH-EPI were compared based on ROIs.
Image analysis: The Ds and Df maps were calculated from the whole brain DW images using a least square nonlinear fitting algorithm in MATLAB (The Mathworks Inc., Natick, MA) according to the following equation. 1:
S=S0*(f*exp(-b*Df) + (1-f)*exp(-b*DS)) [1]
S=S0*exp(-b*ADC) [2]
where Ds is diffusion coefficient, Df is perfusion-related pseudo-diffusion coefficient and f is perfusion fraction. And ADC maps were fitted pixel-wisely from the whole brain according to equation.2.
RESULTS
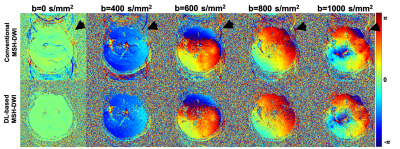
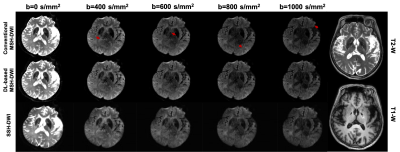
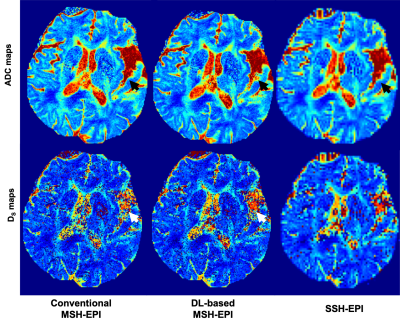
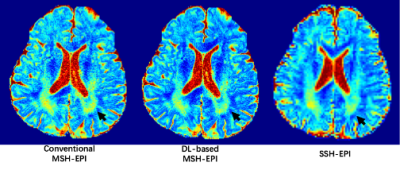
Compared with conventional reconstruction, deep-learning based high resolution DWI showed high advantage in alleviating motion induced artifacts, shown in Fig. 2 (black arrows showed the residual aliasing artifacts in phase results) and Fig.3 (red arrows showed the residual aliasing artifacts in diffusion weighting images) for different b-values (shown with representative high b-values: 400, 600, 800 and 1000 s/mm2) on a patient with old cerebral lacunar infarction. In quantitative comparison, the ADC of deep-learning based method showed clearer boundary for malacia (black arrows in ADC maps of Fig.4 and white arrows in the corresponding DS maps of Fig.4) and infarction lesions (black arrows in Fig.5) compared with conventional method.CONCLUSION
The proposed deep-learning based high resolution IVIM method can qualitatively and quantitatively improve the performance.Acknowledgements
This work was supported by Shanghai Municipal Science and Technology Major Project (No.2017SHZDZX01), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01) and ZJLab, Shanghai Natural Science Foundation (No. 17ZR1401600) and the National Natural Science Foundation of China (No. 81971583).References
1. Markus HS. Cerebral perfusion and stroke. J Neurol Neurosurg Psychiatry. 2004, 75:353-361
.2. Jang M J, et al. Pattern recognition analysis of directional intravoxel incoherent motion MRI in ischemic rodent brains. NMR in Biomedicine, 2020, 33:4268-81.
3. Zhu G, et al. Optimized Combination of b values for IVIM Perfusion Imaging in Acute Ischemic Stroke Patients. Clinical Neuroradiology, 2020, 30:53-544.
4. Brazzelli M, et al. Diffusion-weighted imaging and diagnosis of transient ischemic attack. Annals of Neurology, 2014, 75:67-76.
5. Hotter B, et al. High‐resolution diffusion‐weighted imaging identifies ischemic lesions in a majority of transient ischemic attack patients. Annals of Neurology, 2019, 86:452-457.
6. Chen, N.K., et al. A robust multi-shot scan strategy for high-resolution diffusion weighted MRI enabled by multiplexed sensitivity-encoding (MUSE). Neuroimag, 2013, 72: 41-47.
7. Hui Z, et al. Incorporating Deep Learning into Multi-shot EPI DWI Reconstruction, 2020, the 28th International Society for Magnetic Resonance in Medicine, 1881.
Figures