3236
Functional lung imaging with SENCEFUL using a 2D radial UTE-sequence at different echo times1Department of Diagnostic and Interventional Radiology, University Hospital Würzburg, Würzburg, Germany
Synopsis
MR imaging of the lung is a challenge due to the low proton density in lung parenchyma and the very short relaxation times. To overcome low signal and SNR problems UTE-sequences have successfully been applied to lung imaging. Here, the benefit of a 2D UTE-acquisition for the determination of ventilation and perfusion, as determined by SENCEFUL-MRI, is evaluated. It is shown that SENCEFUL-MRI clearly benefits from the use of a UTE-sequence.
Introduction
MR imaging of the lung is very challenging but of high interest for functional analysis, especially in pediatric patients or to monitor chronic lung diseases. Recently, MR-sequences using ultra-short echo times (UTE-MRI) have been proposed for functional lung imaging (1–3). One approach to determine functional lung parameters is the SENCEFUL algorithm (4), which has already proven itself in multiple clinical applications (1,5,6) and has been combined with 3D UTE to evaluate ventilation values. Here, the benefit of a 2D-UTE-acquisition for the determination of both, ventilation and perfusion, by SENCEFUL-MRI is evaluated.Materials and Methods
All experiments were performed at a 3T-MRI (Siemens MAGNETOM Prisma). Coronal 2D slices in the lung were acquired in 3 healthy volunteers. One Cartesian scan using a quasi-random line ordering was acquired at minimum possible echo time (TE=0.69ms) for comparison. Radial acquisitions with a center-out read-out scheme were acquired at TE= 0.69 ms, 0.40 ms and 0.34 ms. Other imaging parameters were kept constant: TR=2.0ms, flip angle=8°, resolution=3.0x3.0mm2, slice thickness=10mm, measurement time=2min each. In the Cartesian measurement an additional short acquisition window is needed after each read-out to acquire the DC-signal, whereas in the radial scan the first points of each read-out serve as DC-signal. All data was retrospectively gated into 30 respiratory phases and 21 cardiac phases, using the DC-signal. A segmentation of the lung was performed by a region-growing algorithm and functional parameters were determined using the standard SENCEFUL algorithm (4). Ventilation values were normalized to end-expiration signal and overall lung volume change during one breathing cycle. Perfusion values were normalized to a pure blood ROI in the descending aorta. For quality control, the distribution of the ventilation values and the perfusion phase values inside the lung ROI was analyzed.Results
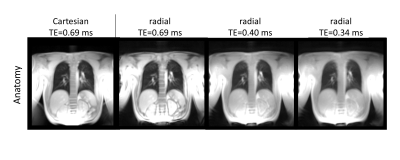
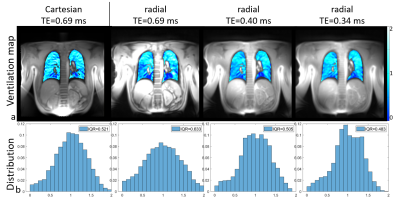
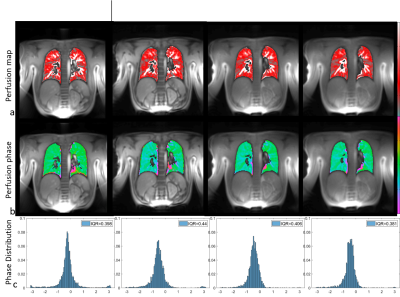
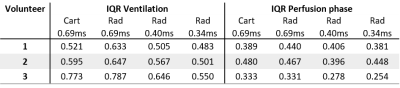
Figure 1, 2 and 3 show results of an exemplary volunteer. Anatomical images in Figure 1 show the expected increase in signal intensity in the lung parenchyma with shorter TE. Also, the overall impression of the images changes as signal intensities approach a pure proton-density weighted contrast and artefacts due to chemical shift disappear. Anatomical images at the same TE, but different read-outs, look similar. The ventilation maps (Figure 2a) appear smoother with decreasing TE. The distribution of ventilation values in the radial acquisitions becomes narrower with shorter TE as shown by the decreasing interquartile range (IQR) (Figure2 b). IQR in a radial acquisition is slightly higher than in the Cartesian acquisition at the same TE. Perfusion maps are comparable through all measurements and echo times (Figure 3a). Large vessels in distal areas are clearly visible, while perfusion values decrease in the proximal regions. Perfusion phase, as deviation from the phase in the pure-blood reference ROI (Figure 3b), appears smooth, large deviations only occur at the edges and close to the heart. The distribution of phase values is small (Figure 3c) and slightly decreases with lower echo times in the radial acquisition. All findings were reproducible in all 3 volunteers, the IQR of ventilation and perfusion phase for all measurements are shown in Table 1.Discussion
Anatomical images show the expected effects of a decreasing echo time. The quantification of ventilation benefits from shorter echo times. In a healthy volunteer, the ventilation is expected to be uniform in a coronal slice, which translates into a small IQR. As the determination is based on a change in signal intensity in the lung parenchyma, the increased signal intensity at short TE render the determination more robust, as supported by the obtained smaller IQR of the ventilation value distribution. Similar results, but weaker effect size, were found for the perfusion maps. This could be caused by the comparatively long T2* relaxation times of blood. For the Cartesian acquisition a minimum echo time of 0.69ms was given by the RF-pulse, slice selection and read-out dephasing times. To achieve shorter TE a radial acquisition was implemented. Unfortunately, these suffer from a non-perfect distribution of sampling points in k-space, leading to lower SNR than in a comparable Cartesian acquisition. Therefore, an initial reduction in the quality of functional parameter maps at similar TE occurs. Nevertheless, the loss can be more than compensated by the shorter TE possible in radial imaging. Additional advantages of radial acquisitions are general robustness towards motion and the implicit acquisition of the DC-signal in each readout. In this study, TR and acquisition time was kept constant for better comparability. However, the included DC signal and the shorter TE of radial acquisitions also would allow for shorter overall measurement times. In contrast to the more common 3D approach for UTE, the 2D acquisition allows the determination of perfusion related parameters. But the necessity of slice selection limits minimum TE, such that a 2D sequence cannot achieve the same shortness of TE as a 3D approach. Additionally, through-plane motion (especially of the heart and large vessels) can cause difficulties in image registration and the determination of functional values.Conclusion
It is shown here that SENCEFUL-MRI clearly benefits from the use of a UTE-sequence. As signal in the lung decays rapidly, shorter echo times can provide increased signal intensity. The improved signal-to-noise consequently allows for a more robust determination of functional parameters.Acknowledgements
No acknowledgement found.References
1. Pereira LM, Wech T, Weng AM, et al. UTE-SENCEFUL: first results for 3D high-resolution lung ventilation imaging. Magnetic Resonance in Medicine 2019;81:2464–2473
2. Balasch A, Metze P, Stumpf K, et al. 2D Ultrashort Echo-Time Functional Lung Imaging. Journal of Magnetic Resonance Imaging 2020;52:1637–1644 doi: https://doi.org/10.1002/jmri.27269.
3. Heidenreich JF, Weng AM, Metz C, et al. Three-dimensional Ultrashort Echo Time MRI for Functional Lung Imaging in Cystic Fibrosis. Radiology 2020;296:191–199
4. Fischer A, Weick S, Ritter CO, et al. SElf-gated Non-Contrast-Enhanced FUnctional Lung imaging (SENCEFUL) using a quasi-random fast low-angle shot (FLASH) sequence and proton MRI. NMR in Biomedicine 2014;27:907–917
5. Kunz A, Weng A, Wirth C, et al. Funktionelle native Lungen-MRT (SENCEFUL) zur Ermittlung pulmonaler Perfusionsdefizite bei Cystischer Fibrose. In: RöFo - Fortschritte auf dem Gebiet der Röntgenstrahlen und der bildgebenden Verfahren. Vol. 189. Georg Thieme Verlag KG; 2017. p. WISS 314.8.
6. Veldhoen S, Weng AM, Knapp J, et al. Self-gated Non–Contrast-enhanced Functional Lung MR Imaging for Quantitative Ventilation Assessment in Patients with Cystic Fibrosis. Radiology 2016;283:242–251
Figures