3231
Free-breathing thoracic imaging using balanced steady-state free precession with half-radial dual-echo readout (bSTAR)1Department of Radiology, Division of Radiological Physics, University of Basel Hospital, Basel, Switzerland, 2Department of Biomedical Engineering, University of Basel, Allschwil, Switzerland
Synopsis
In this work, we demonstrate the application of free-breathing respiratory self-gated thoracic MRI with balanced steady-state free precession half-radial dual-echo imaging technique (bSTAR) in human subjects. The technique combines an efficient minimal-TR readout sampling with an interleaved randomly tilted Archimedean spiral trajectory. The methodological improvements result in high-quality visualization of pulmonary parenchyma and vessel structure. The proposed k-space sampling scheme allows reconstruction of multi-volume data sets at different respiratory phases.
Introduction
Despite the continuous improvement in MR-scanner hardware and data processing techniques, thoracic MRI remains challenging due to the problems associated with physical properties of the lung. Nonetheless, imaging techniques such as ultra-short echo time (UTE)1,2, zero echo time (ZTE)3 or balanced steady-state free precession (bSSFP)4 have shown compelling results in structural and functional lung imaging. More recently, a 3D half-radial dual-echo bSSFP technique known as bSTAR has been demonstrated to provide high-resolution and artifacts-free chest images acquired during a single breath-hold5.The requirement for a prolonged breath-hold, however, can limit the feasibility of the technique especially in children, uncooperative patients, or patients with severe pulmonary disease. Hence, in this work we aimed at the development of a free-breathing bSTAR technique by carefully adapting and exploiting novel k-space sampling strategies merged with respiratory self-gating techniques.
Methods
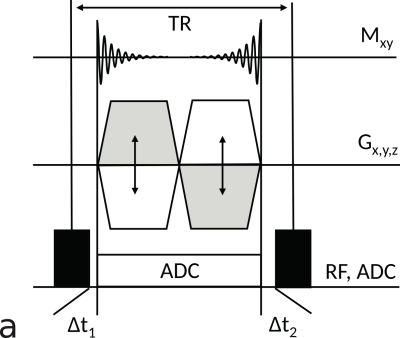
Free-breathing bSTARSimilarly to previously presented techniques5,6, we have implemented a 3D half-radial dual-echo acquisition scheme using a bSSFP kernel with a non-selective rectangular RF excitation pulse and a bipolar readout gradient. In contrast to prior work5, a single ADC (rather than two ADCs) was used along the full bipolar readout to provide not only full maximum sequence efficiency but also improved spatial resolution. Data acquired during each readout comprises a center-out and a center-in half-radial projection. Figure 1 shows the corresponding pulse sequence diagram.
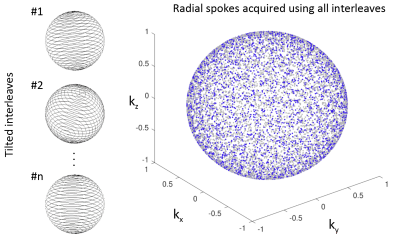
In order to ensure a homogeneous coverage of the k-space over multiple breathing cycles, we have applied short duration interleaves based on Archimedean spirals rotated about the polar axis by the golden angle7. To further mitigate the sampling periodicity with respect to the breathing cycle, each interleave is tilted by a small random polar angle (Figure 2). Furthermore, eddy currents were mitigated by alternating the direction of the consecutive interleaves traversing the k-space along the azimuthal axis, which allows avoiding large jumps in the k-space.
MR data acquisitions
Experiments were performed at 1.5T (MAGNETOM Avanto-Fit, Siemens Healthineers, Erlangen, Germany). Five healthy volunteers (mean age: 36.6 years, range: 27-53 years, three male, two female) were scanned with the proposed free-breathing 3D bSTAR implementation and a single breath-hold 3D bSTAR for comparison. The study was approved by the Institutional Review Board. All scans were performed with predefined shim settings, field-of-view = 35x35x35 cm3, TE1/TE2/TR = 0.11/1.18/1.39ms, 320 samples per half-radial projection, 150us hard RF pulse, flip angle α = 20º, 1856Hz/pixel bandwidth. For free-breathing bSTAR (FB-bSTAR) 240000 half-radial projections were acquired using 240 interleaves, which resulted in scan time of 5.6min. The breath-hold bSTAR (BH-bSTAR) acquisition took 23 seconds with 17000 half-radial projections and 12 interleaves. The pulse sequence efficiency (fraction of TR devoted to sampling the signal) was 0.78.
Image reconstruction
bSTAR datasets were reconstructed off-line using compressed sensing with a fast iterative shrinkage-thresholding algorithm (FISTA-Mod)8. The datasets were reconstructed on a 3843 matrix resulting in 1.4 mm isotropic resolution matching the spatial resolution measured in the k-space. Reconstruction of the breath-hold bSTAR resulted in a single 3D volume. For the reconstruction of the FB-bSTAR, k-space center modulation of a coil located near diaphragm was used to derive the respiratory signal modulation. Readouts acquired during multiple breathing cycles were binned into 10 respiratory phases and reconstructed as separate 3D volumes. The reconstruction pipeline was written in C++ with CUDA Toolkit 11 (NVIDIA Corp. Santa Clara, CA) on a workstation equipped with Quadro P6000 GPU (NVIDIA Corp.).
Results
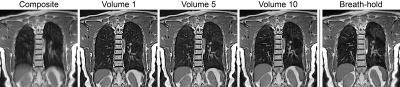
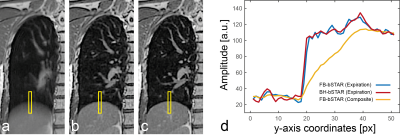
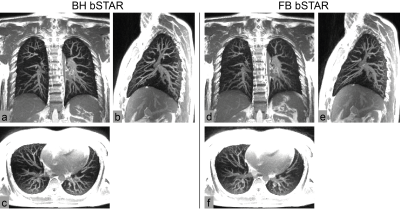
Figure 3 shows exemplary FB-bSTAR reconstructions: a composite image (no gating) along with three different respiratory phases (inspiratory phase, intermediate phase and expiratory phase), as well as an end-expiratory BH-bSTAR acquisition for comparison. The effectiveness of the respiratory self-gating as compared to a breath-hold acquisition is shown in Figure 4. The signal amplitude profile extracted at the diaphragm position from the FB-bSTAR closely matches the profile obtained in BH-bSTAR. Maximum intensity projection images (15mm) acquired in a healthy volunteer using BH-bSTAR and FB-bSTAR are shown in Figure 5. The pulmonary vascular structure is well visualized using both the breath-hold and free-breathing bSTAR acquisitions.Discussion and Conclusion
In this work, we have demonstrated the feasibility of free-breathing 3D bSTAR thoracic imaging with respiratory self-gating in a small group of healthy subjects. Retrospective self-gating represents a major technical improvement of bSTAR imaging and is especially important for less compliant patients. Furthermore, it allows for the reconstruction of different respiratory phases from a single dataset to derive functional parameters related to lung parenchyma density changes or tissue motion. Future studies will focus on the clinical application of our free-breathing bSTAR technique in patients with pulmonary disease.Acknowledgements
No acknowledgement found.References
1. Johnson KM, Fain SB, Schiebler ML, Nagle S. Optimized 3D ultrashort echo time pulmonary MRI. Magn Reson Med 2013;70:1241–1250.
2. Wielpütz MO, Triphan SMF, Ohno Y, et al. Outracing Lung Signal Decay - Potential of Ultrashort Echo Time MRI. Rofo. 2019 May;191(5):415-423.
3. Bae K, Jeon KN, Hwang MJ, et al. Respiratory motion-resolved four-dimensional zero echo time (4D ZTE) lung MRI using retrospective soft gating: feasibility and image quality compared with 3D ZTE. Eur Radiol. 2020 Sep;30(9):5130-5138.
4. Heye T, Sommer G, Miedinger D, et al. Ultrafast 3D balanced steady-state free precession MRI of the lung: Assessment of anatomic details in comparison to low-dose CT. J Magn Reson Imaging. 2015 Sep;42(3):602-9.
5. Bauman G, Bieri O. Balanced steady-state free precession thoracic imaging with half-radial dual-echo readout on smoothly interleaved archimedean spirals. Magn Reson Med. 2020 Jul;84(1):237-246.
6. Diwoky C, Stollberger R. 3D Radial bUTE. In: Proceedings of the 19th Annual Meeting of ISMRM. Montreal; 2011; p. 387.
7. Winkelmann S, Schaeffter T, Koehler T, et al. An optimal radial profile order based on the Golden Ratio for time-resolved MRI. IEEE Trans Med Imaging. 2007 Jan;26(1):68-76.
8. Iliang J, Schoenlieb CB. Improving “Fast Iterative Shrinkage-Thresholding Algorithm”: Faster, Smarter and Greedier. arXiv preprint arXiv:1811.01430
Figures