3230
Phase-Cycled Balanced Steady-State Free Precession Imaging for Functional Lung Imaging at 1.5 and 3 Tesla
Efe Ilicak1, Jascha Zapp1, Safa Ozdemir1, Lothar R. Schad1, and Frank G. Zöllner1,2
1Computer Assisted Clinical Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 2Mannheim Institute for Intelligent Systems in Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany
1Computer Assisted Clinical Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 2Mannheim Institute for Intelligent Systems in Medicine, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany
Synopsis
Functional lung imaging is of great importance for diagnosis of pulmonary diseases. Previously, a non-contrast-enhanced method called Fourier Decomposition was proposed for assessing pulmonary functions based on balanced steady-stated free precession pulse sequence. However, this pulse sequence is known to be sensitive to magnetic field inhomogeneities. Here, we propose a phase-cycled acquisition for improved robustness against field inhomogeneities. In vivo results from 1.5 T and 3 T scanners are provided to demonstrate the performance of phase-cycled acquisitions for functional lung imaging. Preliminary results indicate that phase cycling can be a viable option for reducing limitations arising from magnetic field inhomogeneities.
Introduction
Functional imaging is of great importance for diagnosis and monitoring of prevalent lung diseases. To this end, a non-contrast-enhanced method called Fourier Decomposition (FD) was previously demonstrated to achieve local ventilation and perfusion information during free breathing.1 In FD MRI, a series of images are acquired during free breathing using balanced steady-state free precession (bSSFP) sequence to capture signal variations stemming from respiratory and cardiac signal modulations. Although FD MRI has shown clinical promise, the bSSFP sequence is sensitive to magnetic field inhomogeneities, resulting in irrecoverable signal loss known as banding artefacts.2 Despite significant improvements, occurrence of banding artefacts reduces the robustness of the method, especially hindering its usefulness at a commonly used clinical field strength of 3 T.3,4 In this work, we incorporate RF phase cycling into bSSFP acquisitions to improve FD MRI’s robustness against field inhomogeneities. We present in vivo results to demonstrate the performance of the proposed method.Methods
In FD MRI, a 2D bSSFP sequence with a constant RF phase commonly acquires K images, usually with 3-4 frames/sec.1 Afterwards, this time-series is registered,5 and analysed using temporal Fourier transform to obtain regional density changes corresponding to respiratory and cardiac frequencies. Consequently, ventilation- and perfusion-weighted maps are generated.6In multi-acquisition bSSFP imaging, K images are acquired with N different RF phase increments $$$\Delta\phi_n$$$ with $$$n \in [1 \ N]$$$, to change the spatial location of the banding artefacts.7,8 Here, we have used N=4 different phase cycles with a block-wise phase-cycling scheme,9 meaning that K/4 images were acquired for each phase cycle.
For evaluation, in vivo constant phase and phase-cycled acquisitions were obtained for two volunteers using a 1.5 T scanner (Magnetom Aera, Siemens Healthineers, Erlangen, Germany) with TR/TE = 1.88/0.80 ms, TA = 174 sec; and using a 3 T scanner (Magnetom Skyra, Siemens Healthineers, Erlangen, Germany) with TR/TE = 2.31/1.02 ms, TA = 188 sec. The rest of the parameters were kept identical between the scanners and were as following: FOV = 450 mm x 450 mm, slice thickness = 15 mm, GRAPPA factor = 3, flip angle = 50$$$^\circ$$$, bandwidth = 1302 Hz/Px. For each acquisition, 280 images were acquired using standard shim with a 0.2 s pause between measurements. For the FD analyses, these acquisitions were divided into 4 subgroups (SG) and initial 10 images were excluded to eliminate transient state behavior. Each of these subgroups was then registered individually and the FD was performed to obtain ventilation and perfusion maps of each subgroup. Combinations of these subgroups were also generated.10
To assess the image quality, parenchymal signal-to-noise (SNR) was calculated on registered image series with respect to voxels containing air; and the contrast of ventilation (CNRV) and perfusion (CNRQ) were calculated with respect to their surrounding tissue.
Results
Tables 1 and 2 show the SNR and CNR averaged across the subjects for 1.5 T and 3 T acquisitions. At 1.5 T, $$$\Delta\phi = 0$$$ generates the lowest SNR and CNR scores, meanwhile, $$$\Delta\phi = \pi/2$$$ and $$$\Delta\phi = 3\pi/2$$$ perform similarly in terms of perfusion contrast. At 3 T, the difference of SNR and CNR between the phase cycles diminishes, due to the increased field inhomogeneities.Figure 1 shows the functional maps of each subgroup at 1.5 T for constant phase (a) and phase-cycled (b) acquisitions. As expected, the constant phase acquisition provides similar contrast throughout the experiment whereas phase-cycled acquisition is able to generate functional maps different spatial features. Here, $$$\Delta\phi = \pi$$$ provides more comprehensive perfusion maps in terms of CNR, compared to other phase cycles. Figure 2 shows the functional maps of each subgroup at 3 T. Here, conventional $$$\Delta\phi = \pi$$$ acquisition suffers from increased field inhomogeneities, especially visible in the perfusion maps. Whereas, with RF phase cycling, the proposed method improves robustness against field inhomogeneities and is able to obtain more comprehensive perfusion maps.
Figure 3 shows the combined maps overlaid on top of a single cross-section for 1.5 T (a) and 3 T (b) acquisitions. At 1.5 T, phase-cycled acquisition suffers from lower signal values in the perfusion maps due to averaging effects. Nonetheless, phase-cycled maps provide similar contrast and prominent structures compared to their constant phase version. At 3 T, banding artefacts are more prominent due to increased field inhomogeneity as expected, and the constant phase acquisition is less able to reproduce structures and a comprehensive perfusion map. On the other hand, the phase-cycled acquisitions are able to improve the robustness against field inhomogeneity and reproduce prominent structures and a comprehensive perfusion map.
Discussion & Conclusion
We have developed and demonstrated phase-cycled bSSFP acquisitions for functional lung imaging. Individual and combined ventilation- and perfusion-weighted maps were successfully displayed at 1.5 T and 3 T field strengths. While further studies with more subjects are warranted, our preliminary results indicate that phase cycling can be incorporated to FD MRI to improve robustness against magnetic field inhomogeneities, at the expense of reduced scan efficiency. Overall, phase cycling can be a viable option for reducing limitations arising from magnetic field inhomogeneities and therefore, increase the clinical value of FD MRI.Acknowledgements
This work was supported by Deutsche Forschungsgemeinschaft (grant number: DFG 397806429).References
- Bauman G, Puderbach M, Deimling M, et al. Non-contrast-enhanced perfusion and ventilation assessment of the human lung by means of Fourier decomposition in proton MRI. Magn Reson Med. 2009;62(3):656-664. doi:10.1002/mrm.22031
- Bangerter NK, Hargreaves BA, Vasanawala SS, et al. Analysis of multiple-acquisition SSFP. Magn Reson Med. 2004;51(5):1038-1047.
- Bauman G, Pusterla O, Bieri O. Ultra-fast Steady-State Free Precession Pulse Sequence for Fourier Decomposition Pulmonary MRI. Magn Reson Med. 2016;75(4):1647-1653. doi:10.1002/mrm.25697
- Bauman G, Pusterla O, Bieri O. Functional lung imaging with transient spoiled gradient echo. Magn Reson Med. October 2018. doi:10.1002/mrm.27535
- Chefd’hotel C, Hermosillo G, Faugeras O. Flows of diffeomorphisms for multimodal image registration. In: Proceedings IEEE International Symposium on Biomedical Imaging. IEEE; :753-756. doi:10.1109/ISBI.2002.1029367
- Ilicak E, Chacon-Caldera J, Zapp J, Schad LR, Zoellner FG. Compressed Sensing reconstruction in non-contrast-enhanced functional lung MRI using Fourier Decomposition: An initial study. Proc Intl Soc Mag Reson Med. 2019;27:1897.
- Çukur T. Accelerated Phase-Cycled SSFP Imaging With Compressed Sensing. IEEE Trans Med Imaging. 2015;34(1):107-115. doi:10.1109/TMI.2014.2346814
- Lauzon ML, Frayne R. Analytical characterization of RF phase-cycled balanced steady-state free precession. Concepts Magn Reson. 2009;34A(3):133-143.
- Benkert T, Ehses P, Blaimer M, Jakob PM, Breuer FA. Dynamically phase-cycled radial balanced SSFP imaging for efficient banding removal. Magn Reson Med. 2015;73(1):182-194.
- Brennan DG. Linear Diversity Combining Techniques. Proc IRE. 1959;47(6):1075-1102. doi:10.1109/JRPROC.1959.287136
Figures
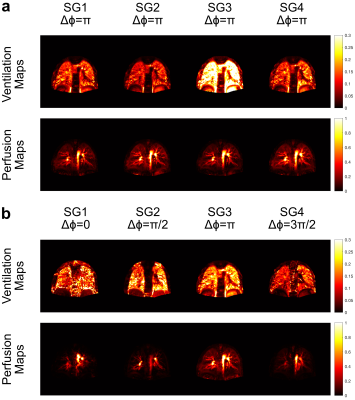
Figure 1:
Ventilation and perfusion maps of subgroups at 1.5 T for constant phase (a) and
phase-cycled (b) bSSFP acquisitions. As expected, constant phase acquisition
generates similar functional maps throughout the experiment whereas
phase-cycled acquisition is able to generate functional maps with different
information by changing the RF phase. At 1.5 T, the conventional $$$\Delta\phi = \pi$$$ provides more
comprehensive perfusion maps compared to other phase cycles, but phase-cycled
acquisition is still able to capture this information.
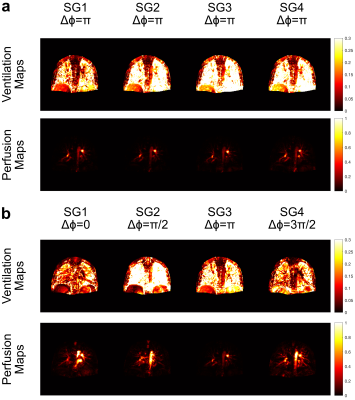
Figure 2:
Ventilation and perfusion maps of subgroups at 3 T for constant phase (a) and
phase-cycled (b) bSSFP acquisitions. As expected, constant phase acquisition
generates similar functional maps throughout the experiment whereas
phase-cycled acquisition is able to generate functional maps with different
information by changing the RF phase. At 3 T, the perfusion maps with
conventional $$$\Delta\phi = \pi$$$ acquisition suffer
from field inhomogeneity and are less comprehensive compared to phase-cycled
acquisitions.
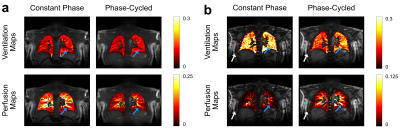
Figure
3: Combined functional maps overlaid on
a cross-section shown for 1.5 T (a) and 3 T (b). At 1.5T, both phase-cycled maps
provide similar contrast and prominent structures (blue arrows) compared to
constant phase maps while phase-cycled perfusion map suffers from overall lower
values due to averaging. At 3T, both phase-cycled
maps show similar contrast and prominent structures (blue arrows) compared to
1.5T. However, the constant phase is less able to reproduce these structures since
it is more prone to field
inhomogeneity artefacts
(white arrows).
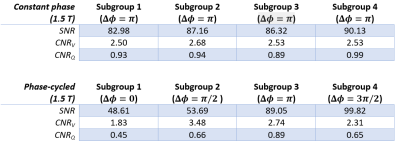
Table 1: Signal-to-noise
ratio (SNR) of registered images and contrast measurements (CNR) of functional
maps are reported at 1.5 T for constant phase and phase-cycled measurements
across subgroups (mean across subjects).
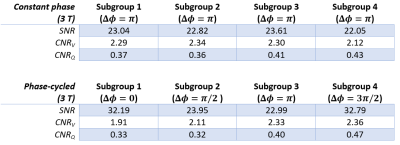
Table 2:
Signal-to-noise ratio (SNR) of registered images and contrast measurements
(CNR) of functional maps are reported at 3 T for constant phase and
phase-cycled measurements across subgroups (mean across subjects).