3228
A 3D Stack-of Spirals Approach for Inert Fluorinated (19F) Gas MRI of the Lungs1Translational Medicine, The Hospital for Sick Children, Toronto, ON, Canada, 2Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Siemens Healthcare Limited, Montreal, QC, Canada, 4McConnell Brain Imaging Centre, Montreal Neurological Institute, Montreal, QC, Canada, 5Department of Neurology and Neurosurgery, McGill University, Montreal, QC, Canada
Synopsis
Inert fluorinated (19F) gas MRI is an emergent technology that has potential to be a lower cost, reduced infrastructure alternative to hyperpolarized gas MRI for lung imaging. In this work we present an approach for non-Cartesian spiral imaging in a phantom. Compared to typical gradient echo imaging with the same spatial resolution, the proposed approach increased SNR by 44% in approximately a third of the acquisition time. Further simulation and extrapolation to clinical imaging experiments indicate significant potential for this approach to improve SNR and image quality.
Introduction
Inert fluorinated (19F) gas MRI is an emergent technology that has potential to be a lower cost, reduced infrastructure alternative to hyperpolarized (HP) gas MRI for lung imaging1, providing similar and complementary information2,3. These gases, such as sulfur hexafluoride (SF6) or perfluoropropane (PFP; C3F8), are biologically inert, low cost, and have favorable properties for MRI. Most importantly, they do not require hyperpolarization, instead relying on a relatively high number of 19F nuclei per molecule and signal averaging; enabling imaging under thermal equilibrium conditions1.19F gas MRI is typically performed with gradient echo (GRE) or steady-state free precession (SSFP) imaging using cartesian approaches, resulting in modest resolutions and SNR due to the inherently low signal intensities and short T2*. Non-Cartesian sequences (e.g. spiral k-space trajectories) are rapid, and more efficiently cover k-space possibly allowing for more signal averages during a typical participant breath-hold (≤20 sec), while also potentially permitting reduced echo times2,4.
In this preliminary work, we theoretically and experimentally compare 2D-GRE with rapid, non-Cartesian 19F gas imaging using a 3D stack-of-spirals (3D-SoS) pulse sequence5.
Methods
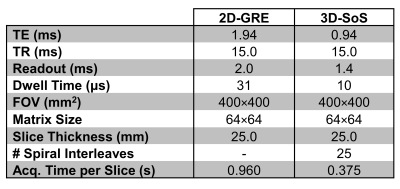
Imaging was performed on a clinical 3T scanner (MAGNETOM Prisma, Siemens, Erlangen, Germany). A 1 L bag was filled with SF6 (Messer Canada, Mississauga, Canada) and imaged using a 19F birdcage transmitter and 8-channel receive array (Rapid Biomedical, Rimpar, Germany). Table 1 shows the acquisition parameters. The spatial resolution for both sequences was 6.25×6.25×25 mm3. Transmitter voltage was adjusted to yield a 90⁰ flip angle and both acquisitions were averaged 10 times for sufficient signal intensity. Images were reconstructed in MATLAB (MathWorks, Natick, MA). 3D-SoS images were reconstructed using a non-uniform FFT6. A 9×9 pixel ROI was used to estimate SNR (Fig. 1). A simulation comparing the achievable scan durations of both sequences for several in-plane spatial resolutions, assuming 10 slices, and keeping all other parameters in Table 1 constant, was performed. A minimum TR 3D-SoS case for each resolution was also explored. This was extended to estimate the number of signal averages (NSA) achievable in a 20 sec breath-hold. Finally, the relative SNR changes (normalized to the 64x64 2D-GRE case) accounting for differences in TE, in-plane resolutions, and number of achievable signal averages was simulated under assumed Ernst angle imaging conditions for PFP (T1=12 ms and T2*=2 ms)7.Results
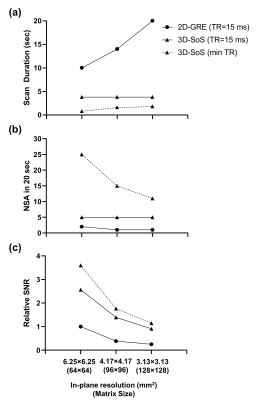
From Table 1 it is observed that with these parameters 3D-SoS is 2.56 times faster per slice than 2D-GRE. Fig. 1 shows images acquired with both sequences. 3D-SoS images are observed to have image quality comparable to 2D-GRE. However, SNR was observed to be increased using 3D-SoS compared to 2D-GRE with values of 19.5 and 13.6, respectively. Extrapolating these results to clinical imaging (Fig. 2) with simulation demonstrates the advantage of fast, spiral imaging to reduce scan duration and increase the NSA in a breath-hold, especially if short TR can be maintained.Discussion
In this work a 3D-SoS acquisition is applied to 19F gas imaging in a phantom. 3D-SoS demonstrated a 44% improvement in SNR versus 2D-GRE for the same spatial resolution and number of signal averages, in nearly one third of the time. This is presumably due to the decreased minimum TE of the 3D-SoS sequence resultant from the spiral-out trajectory. Further reductions in TE should be achievable with shorter RF pulse durations, variable TE in kz, or fully non-selective acquisitions. Stack-of-stars UTE has demonstrated improved SNR with 19F gas MRI4. However, in that study, long acquisition windows and in-plane radial undersampling in order to comply with breath-hold constraints contributed to significant image blur, negating the advantages of reduced TE. Here, fully-sampled data with comparatively few acquisitions and short readouts is possible due to the efficiency of the spiral k-space trajectory, mitigating image corruption while reducing TE.Although this work is very preliminary and limited to phantoms, there is significant promise for clinical use. Fig. 2a illustrates simulated achievable scan durations for several in-plane resolutions. These illustrate the significant reduction in scan duration and potential for increased NSA with 3D-SoS (Fig. 2b) leading to increased SNR (Fig. 2c). Furthermore, it is observed that SNR improvements may be traded for improved resolution. Alternatively, reductions in scan time may be leveraged to perform rapid, repeated imaging during extended multi-breath or free-breathing experiments to explore gas kinetics, since 19F gases may be freely administered in normoxic mixtures8–10. However, these simulations, especially the minimum TR case, should be interpreted with caution, requiring experimental corroboration and may represent an idealized scenario. Human loading conditions may limit achievable RF power and/or such short TR may exceed SAR thresholds. Nevertheless, there is likely a “practical middle-ground” that may be empirically determined.
Lastly, this work uses SF6 which may be less desirable than PFP due to the increased RF deposition requirements to maintain Ernst angle imaging associated with a short T1 of ~1 ms. PFP has a more favorable T1 (12 ms) reducing RF transmit requirements while remaining short enough to maintain rapid, repeated imaging and will be used in future clinical studies.
Conclusion
3D-SoS allows for improved SNR compared to 2D-GRE under similar imaging conditions. Extrapolating to clinical imaging indicates the potential of this acquisition to improve 19F gas MRI in humans.Acknowledgements
Funding was provided by the Natural Sciences and Engineering Research Council (NSERC) of Canada.References
1. Couch MJ, Ball IK, Li T, Fox MS, Biman B, Albert MS. 19 F MRI of the Lungs Using Inert Fluorinated Gases: Challenges and New Developments. J Magn Reson Imaging. 2019;49(2):343-354. doi:10.1002/jmri.26292
2. Obert AJ, Gutberlet M, Kern AL, et al. 1H-guided reconstruction of 19F gas MRI in COPD patients. Magn Reson Med. 2020;84(3):1336-1346. doi:10.1002/mrm.28209
3. McCallister A, Chung SH, Antonacci M, et al. Comparison of single breath hyperpolarized 129 Xe MRI with dynamic 19 F MRI in cystic fibrosis lung disease. Magn Reson Med. 2020;0(0):1-11. doi:10.1002/mrm.28457
4. Couch MJ, Ball IK, Li T, et al. Pulmonary ultrashort echo time 19F MR imaging with inhaled fluorinated gas mixtures in healthy volunteers: Feasibility. Radiology. 2013;269(3):903-909. doi:10.1148/radiol.13130609
5. Zanette B, Friedlander Y, Munidasa S, Santyr G. Comparison of 3D Stack-of-Spirals and 2D Gradient Echo for Ventilation Mapping using Hyperpolarized 129Xe. Proc Intl Soc Mag Reson Med. 2020;28:0449.
6. Fessler JA, Member S, Sutton BP. Nonuniform Fast Fourier Transforms Using Min-Max Interpolation. IEEE Trans Signal Process. 2003;51(2):560-574.
7. Couch MJ, Ball IK, Li T, et al. Inert fluorinated gas MRI: a new pulmonary imaging modality. NMR Biomed. 2014;27(12):1525-1534. doi:10.1002/nbm.3165
8. Gutberlet M, Kaireit TF, Voskrebenzev A, et al. Free-breathing dynamic 19F gas MR imaging for mapping of regional lung ventilation in patients with COPD. Radiology. 2018;286(3):1040-1051. doi:10.1148/radiol.2017170591
9. Gutberlet M, Kaireit TF, Voskrebenzev A, et al. Repeatability of Regional Lung Ventilation Quantification Using Fluorinated (19F) Gas Magnetic Resonance Imaging. Acad Radiol. 2019;26(3):395-403. doi:10.1016/j.acra.2018.10.021
10. Goralski JL, SH C, Glass T, et al. Dynamic perfluorinated gas MRI reveals abnormal ventilation despite normal FEV1 in cystic fibrosis. JCI Insight. 2019:E-Pub Ahead of Print.
Figures