3226
Simultaneous segmentation of airways and ventilated lung in hyperpolarised-gas MR images by deep learning1Télécom SudParis, Paris, France, 2POLARIS, Department of Infection, Immunity and Cardiovascular Disease, The University of Sheffield, Sheffield, United Kingdom, 3Insigneo Institute for in silico Medicine, The University of Sheffield, Sheffield, United Kingdom
Synopsis
The assessment of pulmonary hyperpolarised(HP)-gas MR images is instrumental in identifying potential pathologies, directing treatment, or monitoring disease progression. HP-gas images quantify the amount of gas concentration, whose distribution within the lungs is analysed. A key role in this process is played the main airways that are identified for quality control and excluded for the analysis. Currently, this task is performed manually and existing deep-learning (DL) applications do not provide an explicit labelling of the airways. A specific tailoring of a well-known DL approach was developed with the aim of replacing the manual editing thanks to its good performance.
Introduction
The assessment of pulmonary hyperpolarised-gas MR images is instrumental in identifying potential pathologies1-3, directing treatment4, or monitoring disease progression5. The signal in HP gas images is proportional to gas concentration; in turn, the value distribution within the lung region is analysed to produce a ventilation grading (binary6 or multi-class, e.g. linear binning maps7) and, where possible, a treatment response mapping8. A key step in this process is the segmentation of the ventilated regions, where, among other tasks, the main airways are identified, excluded from the lung cavity region for analysis purposes, and highlighted for quality control. Currently, this task is performed manually, takes valuable time from the image assessors, and existing deep-learning (DL) applications9,10 do not provide an explicit labelling of the airways. To address this need, a specific tailoring of a well-known DL approach11 to image segmentation was developed. The solution is aimed at replacing the need for manual editing due to its good performance in discriminating the ambiguous features between airways and ventilated lung regionsMaterials
All MR images were obtained with a 1.5-T whole-body system (Signa HDx; GE Healthcare) and a 129Xe transmit-receive vest coil (CMRS). Images were acquired coronally during breath hold using a 3D steady state free precession sequence10 with subjects in the supine position. The training subset consisted of 167 3D images. The testing set consisted of 28 3D images from unique subjects that were not present in the training set. For each image in the training and testing subsets, ground truth images were manually edited by experienced readers.Methods
SegmentationA three-class DL-based segmentation was implemented using the MONAI framework13 to identify background, ventilated parenchyma, and airways voxels. A 3D UNet architecture11 with 2 residual units was selected because of its flexibility in customising the channel configuration; in our case a network hierarchy with 4 layers of 32, 64, 128, and 512 channels, respectively, was used. Inference was performed by a sliding window approach13 with 32 iterations. A specific loss function was created by adding contributions from a Sørensen-Dice similarity14 (SDS) coefficient loss term, a cross entropy loss15 term, and a newly devised penalty term proportional to the amount of lung ventilation erroneously labelled as airways.
Analysis
Segmentation performance was assessed by computing the SDS coefficient between the predicted and the ground-truth segmentations of the ventilated-lung and airways region, respectively. Median and interquartile range (IQR) of these coefficients were then determined. Additionally, in order to estimate the amount of disagreement with the ground truth, the XOR metric16 was computed on the airways region as follows $$\mathrm{XOR} = \frac{\left|P\cap G^{\prime}\right|+\left|P^{\prime}\cap G\right|}{\left|G\right|}$$where P is the predicted region, G is the ground truth, and prime indicates complement.
Results
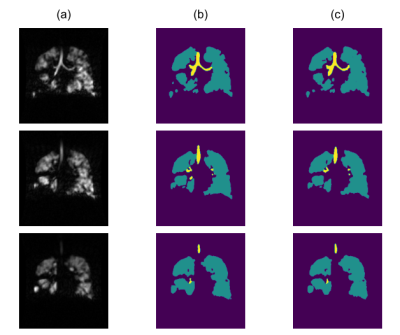
The selected model was computed after 592 epochs. Median SDS coefficient for the airways segmentation was 88.5% (IQR=9.6%). Median SDS coefficient for the ventilated-lung segmentation was 97.5% (IQR=1.0%). Median XOR value for the airways regions was 22.6% (IQR=16.8%). Examples of automated segmentations are shown in Figures 1 and 2.Discussion
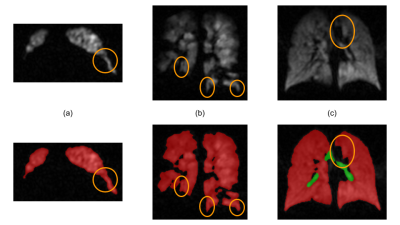
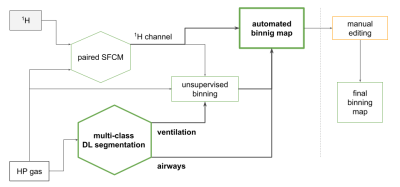
In order to assess the HP-gas ventilation images, the ventilation grading information can be provided by binary (as in 6) or multi-class classification, e.g. the linear binning clustering technique7. In both cases, the extra-pulmonary regions must be excluded from the subsequent analysis and the identification of the airways is an essential component of this process. Automation of this task is therefore important with it being beneficial to provide assessors with an auditable outcome for the quality control. The segmentation of the airways is particularly challenging because of the possible ambiguities with some lung regions, as shown in Figure 2, and the large size imbalance with the ventilated-lung regions. Ambiguities occur in small areas and they can be broadly classified in two main categories: (1) those appearing like a tubular structure (Figure 2.a) or a high-signal portion of the trachea (Figure 2.b), but whose location is far away from the typical location of the main airways, and (2) in cases like Figure 2.c, when we are presented with an inner-border portion of ventilated lung appearing as a medium-signal airway tract. We tackled the first issue by increasing the number of channels at the base layer in order to capture a larger portion of the geometry surrounding each voxel. As regards the second category, we minimised the lung-as-airways mislabelling by defining an additional loss term that would greatly penalise each of those errors. The combination of those architectural factors resulted in a good segmentation performance. Additionally, the low values of the XOR metric are a clear indication that the amount of manual correction required after DL segmentation, even at this initial stage of the development, is minimal.Figure 3 shows how the binning map workflow, which already relied on the SFCM segmentation17,18, can be further automated with a dependable identification of airways and lung ventilated regions.
Conclusion
The DL-based simultaneous segmentation of airways and ventilated lung regions correctly discriminated the two labels, even in the presence of similar features reducing the time needed to segment ventilation images. Due to its good performance, this new segmentation approach has been included in the linear binning workflow7 for the routine assessment of HP gas ventilation MR imagesAcknowledgements
No acknowledgement found.References
1. Kirby M, Svenningsen S, Owrangi A, Wheatley A, Farag A, Ouriadov A, Santyr GE, Etemad-Rezai R, Coxson HO, McCormack DG, Parraga G.Hyperpolarized 3He and 129Xe MR imaging in healthy volunteers and patients with chronic obstructive pulmonary disease. Radiology 2012;265(2):600-610.
2. Marshall H, Horsley A, Taylor CJ, Smith L, Hughes D, Horn FC, Swift AJ, Parra-Robles J, Hughes PJ, Norquay G, Stewart NJ. Detection of early subclinical lung disease in children with cystic fibrosis by lung ventilation imaging with hyperpolarised gas MRI. Thorax. 2017 Aug 1;72(8):760-2.
3. Salerno M, Altes TA, Mugler III JP, Nakatsu M, Hatabu H, de Lange EE. Hyperpolarized noble gas MR imaging of the lung: potential clinical applications. European journal of radiology. 2001 Oct 1;40(1):33-44.
4. Ireland RH, Tahir BA, Wild JM, Lee CE, Hatton MQ. Functional image-guided radiotherapy planning for normal lung avoidance. Clinical Oncology. 2016 Nov 1;28(11):695-707.
5. Smith L, Marshall H, Aldag I, Horn F, Collier G, Hughes D, West N, Horsley A, Taylor CJ, Wild J. Longitudinal assessment of children with mild cystic fibrosis using hyperpolarized gas lung magnetic resonance imaging and lung clearance index. American journal of respiratory and critical care medicine. 2018 Feb 1;197(3):397-400.
6. Woodhouse N, Wild JM, Paley MN, Fichele S, Said Z, Swift AJ, van Beek EJ. Combined helium‐3/proton magnetic resonance imaging measurement of ventilated lung volumes in smokers compared to never‐smokers. Journal of Magnetic Resonance Imaging. 2005 Apr;21(4):365-9.
7. Collier G et al. Linear binning maps for image analysis of pulmonary ventilation with hyperpolarized gas MRI: transferability and clinical applications. Proc. ISMRM Meeting'18, abstract 4482, 2018.
8. Collier, GJ, Biancardi AM, Hughes PJ, Smith L, Mussel GT, Marshall H, Chan HF, Norquay G, Wild J. Binning method for treatment response mapping with hyperpolarized gas lung MRI: application in subjects with asthma. Proc. ISMRM Meeting'20, abstract 0442, 2020.
9. Tustison NJ, Avants BB, Lin Z, Feng X, Cullen N, Mata JF, Flors L, Gee JC, Altes TA, Mugler III JP, Qing K. Convolutional neural networks with template-based data augmentation for functional lung image quantification. Academic radiology. 2019 Mar 1;26(3):412-23.
10. Astley JR, Biancardi AM, Hughes PJ, Smith LJ, Marshall H, Eaden J, Bray J, Weatherley ND, Collier GJ, Wild JM, Tahir BA. 3D deep convolutional neural network-based ventilated lung segmentation using multi-nuclear hyperpolarized gas MRI. In International Workshop on Thoracic Image Analysis 2020 Oct 8 (pp. 24-35). Springer, Cham.
11. Kerfoot E, Clough J, Oksuz I, Lee J, King AP, Schnabel JA. Left-ventricle quantification using residual U-Net. In International Workshop on Statistical Atlases and Computational Models of the Heart 2018 Sep 16 (pp. 371-380). Springer, Cham.
12. Stewart NJ, Norquay G, Griffiths PD, Wild JM. Feasibility of human lung ventilation imaging using highly polarized naturally abundant xenon and optimized three-dimensional steady-state free precession. Magn Reson Med 2015;74:346-52.
13. Project MONAI. Project MONAI - Medical Open Network for AI. https://monai.io/
14. Wikipedia. https://en.wikipedia.org/wiki/S%C3%B8rensen%E2%80%93Dice_coefficient
15. PyTorch. https://pytorch.org/docs/stable/generated/torch.nn.CrossEntropyLoss.html
16. Biancardi AM, Wild JM. New Disagreement Metrics Incorporating Spatial Detail–Applications to Lung Imaging. In: Annual Conference on Medical Image Understanding and Analysis 2017 Jul 11 (pp. 804-814). Springer, Cham.
17. Hughes PJ, Horn FC, Collier GJ, Biancardi A, Marshall H, Wild JM. Spatial fuzzy c‐means thresholding for semiautomated calculation of percentage lung ventilated volume from hyperpolarized gas and 1H MRI. Journal of Magnetic Resonance Imaging. 2018 Mar;47(3):640-6.
18. Biancardi A, Acunzo L, Marshall H, Tahir BA, Hughes PJ, Smith L, Weatherley ND, Collier GJ, Wild J. A paired approach to the segmentation of proton and hyperpolarized gas MR images of the lungs. Proc. ISMRM Meeting'18, abstract 2442, 2018.
Figures