3222
Accelerated dynamic 19F-MRI of inhaled perfluoropropane for quantitative regional assessment of gas wash-in biomarkers in patients with COPD
Mary A. Neal1,2, Benjamin J. Pippard1,2, Kieren G. Hollingsworth1,2, A. John Simpson2, and Peter E. Thelwall1,2
1Newcastle Magnetic Resonance Centre, Newcastle University, Newcastle upon Tyne, United Kingdom, 2Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, United Kingdom
1Newcastle Magnetic Resonance Centre, Newcastle University, Newcastle upon Tyne, United Kingdom, 2Translational and Clinical Research Institute, Newcastle University, Newcastle upon Tyne, United Kingdom
Synopsis
19F-MRI of inhaled perfluoropropane (PFP) permits regional assessment of pulmonary ventilation. Dynamic multi-breath PFP wash-in and wash-out images were acquired in one healthy volunteer and two patients with chronic obstructive pulmonary disease (COPD) during short successive breath holds, using a repeated 3D 19F-SPGR sequence accelerated with compressed sensing. Resultant image quality achieved in each 7.5s compressed sensing acquisition dynamic was sufficient for segmentation and permitted visualisation of a case of gas trapping secondary to apical predominant emphysema. A strong negative correlation between regional wash-in and wash-out rates and spirometric indices was measured.
Introduction
MRI of inhaled MR-visible gas agents permits direct visualization of gas distribution for regional assessment of pulmonary ventilation. Static breath hold ventilation MRI captures signal distribution at a fixed time-point, disregarding the progressive gas turnover and wash-in which occurs over multiple breaths. This risks insensitivity to detection of delayed ventilation in lung regions distal to diseased airways. Dynamic MR imaging of gas wash-in over multiple breaths permits analysis of the evolving signal distribution for quantification of regional time-domain biomarkers of ventilation.Hyperpolarized gas MRI (HP-MRI) is a mature area of research with established utility for assessment of pulmonary ventilation, predominantly using static breath hold imaging techniques.1-4 Literature featuring dynamic HP-MR imaging of multi-breath wash-in is sparse.5,6 The oxygen-dependent depolarization rate convolutes dynamic image acquisition and analysis; and the requirement for gas polarizing equipment presents a possible barrier to widespread clinical application.
Perfluoropropane (PFP) is an inert, commercially available and comparably inexpensive MR-visible gas which does not require hyperpolarization. The utility of static 19F-MR imaging of inhaled PFP for assessment of ventilation defects has been demonstrated in healthy volunteers7,8 and patients with lung disease.9,10 Its suitability for dynamic imaging of multi-breath wash-in has recently been demonstrated.11,12
We assess the capability of a dynamic multi-breath 19F-MR imaging technique, accelerated with compressed sensing, for quantitative assessment of gas wash-in and wash-out rates in vivo.
Methods
HRA ethical approval was granted following review by the Newcastle and North Tyneside 2 Research Ethics Committee (Ref: 18/NE/0297) and the NHS Health Research Authority.One healthy volunteer (HV1: female, aged 31) and two patients with COPD (P1: male, aged 68; P2: male, aged 76) were screened for study eligibility and provided written informed consent.
All participants underwent a single MRI scan session with a same-day spirometry test. MR images were acquired using a 19F/1H birdcage torso coil (Rapid Biomedical, Germany) interfaced to a Philips 3T Achieva scanner. A gas delivery system with a manually operated two-way valve delivered room air or a 79% PFP/21% O2 gas mixture (PFP/O2) via a mouthpiece during the scan session.
Participants were instructed to perform a deep inhalation of PFP/O2 before commencement of a breath hold. During the breath hold, a 3D SPGR 19F-MRI compressed sensing (CS) acquisition was performed (TE=1.7ms, TR=8.5ms, flip angle=45°, FOV=400×320×250mm3, resolution=10×10×10mm3, bandwidth=500Hz/pixel, averages=3, CS acceleration factor=1.8, acquisition time=7.5s).13 An 'exhale-inhale-exhale-inhale-breath hold' manoeuvre was then repeated for a maximum of 10 breath hold acquisitions, or until near-complete PFP wash-in was visualised on the scanner interface. The gas delivery system was then switched to room air to permit imaging of PFP/O2 wash-out whilst employing the same breathing manoeuvres.
3D 1H anatomical images (TE=0.49ms, TR=4ms, flip angle=6°, FOV=440×440×247.5mm3, resolution=3×3×7.5mm3, bandwidth=3400Hz/pixel, acquisition time=14.6s) were acquired in a separate breath hold at maximum inspiration.
Images were segmented using intensity-based signal thresholding. Anatomical 1H images were registered to each dynamic of the 19F-MR image sets for calculation of percent ventilated lung volume (%VV).
Monoexponential recovery and decay curves, with time-constants τ1 and τ2 respectively and units of respiratory cycles (describing the number or breaths required to achieve ~63.2% of the calculated maximum signal during wash-in, or a reduction to ~36.8% of the maximum value during wash-out), were fitted to the signal measured at corresponding 19F-MR image voxels in the time-domain.
Neighbourhood τ1 and τ2 was measured by segmenting the lung volumes into octants. Correlation between median neighbourhood τ and spirometric forced expiratory volume in the first second (FEV1) was measured using the Pearson correlation coefficient.
Results
19F-MRI was well tolerated by all participants with no adverse events. The 19F-MR acquisition for P1 was halted prior to collection of wash-out data due to an operational error.Figure 1 displays coronal 19F-MR image slices depicting PFP signal wash-in and wash-out for each volunteer. Substantial apical gas trapping was noted in P2. Figure 2 displays %VV calculated from each breath hold acquisition.
Figure 3 displays voxel-wise τ1 and τ2 maps for each participant. Median τ1 measured in each quartile was 1.5(range: 1.1-2.2) respiratory cycles in HV1, 3.1(2.2-4.3) in P1 and 4.8(3.4-7.1) in P2. A strong negative correlation was found between median τ1 and FEV1 (r=-0.79). Median τ2 was measured in each octant as 1.5(1.0-1.8) in HV1 and 5.3(3.7-12.0) in P2; (r=-0.79). Figure 4 displays global and neighbourhood τ1 and τ2 distributions.
Discussion
Image quality from the 7.5s-duration CS acquisitions was sufficient for segmentation and subsequent analysis of gas wash-in and wash-out using a thermally polarised tracer gas. To our knowledge, this work presents the first direct dynamic visualisation of gas trapping secondary to apical predominant emphysema, with localised apical gas trapping visible after successive deep wash-out breaths in P2.Pixel-wise and neighbourhood assessment of gas distribution revealed an increased minimum, median and range of τ1 and τ2 in the patient datasets compared to healthy control data, and strong correlations with FEV1.
Conclusion
Dynamic accelerated multi-breath 19F-MRI of inhaled PFP was performed with image quality adequate for segmentation and calculation of %VV. Pixel-wise and neighbourhood wash-in and wash-out rates were assessed and revealed a strong correlation with spirometric indices.Acknowledgements
Advice and technical support from Dr Matthew Clemence (Philips Healthcare) are gratefully acknowledged.References
- Pike D, Kirby M, Guo F, McCormack DG, Parraga G. Ventilation heterogeneity in ex-smokers without airflow limitation. Acad Radiol. 2015;22(8):1068–1078.
- O’Sullivan B, Couch M, Roche JP, et al. Assessment of repeatability of hyperpolarized gas MR ventilation functional imaging in cystic fibrosis. Acad Radiol. 2014;21(12):1524–1529.
- Ebner L, He M, Virgincar RS, et al. Hyperpolarized 129Xenon magnetic resonance imaging to quantify regional ventilation differences in mild to moderate asthma: a prospective comparison between semiautomated ventilation defect percentage calculation and pulmonary function tests. Invest Radiol. 2017;52(2):120–127.
- Ouriadov A, Farag A, Kirby M, McCormacck DG, Parraga G, Santyr GE. Lung morphometry using hyperpolarized 129Xe apparent diffusion coefficient anisotropy in chronic obstructive pulmonary disease. Magn Reson Med. 2013;70(6):1699–1706.5.
- Hamedani H, Clapp JT, Kadlecek SJ, et al. Regional fractional ventilation by using multibreath wash-in 3He MR imaging. Radiology. 2016;279(3):917–924.
- Horn FC, Deppe MH, Marshall H, Parra-Robles J, Wild JM. Quantification of regional fractional ventilation in human subjects by measurement of hyperpolarized 3He washout with 2D and 3D MRI. J Appl Physiol. 2014;116(2):129–139.
- Couch MJ, Ball IK, Li T, Fox MS, Biman D, Albert MS. 19F MRI of the lungs using inert fluorinated gases: challenges and new developments. J Magn Reson Imaging. 2019;49(2):343–354.
- Neal MA, Pippard BJ, Maunder A, et al. 19F-MRI of inhaled perfluoropropane for assessment of pulmonary ventilation: a multi-centre reproducibility study in healthy volunteers. In Proceedings of the 28th Annual Meeting of ISMRM, Online Virtual Conference, 2020. Abstract number 0440.
- Pippard B, Neal M, Maunder A, et al. Assessing regional lung ventilation with 19F-MRI of inhaled perfluoropropane. Eur Respir J. 2018. doi: 10.1183/13993003.congress-2018.OA3799.
- Halaweish AF, Moon RE, Foster WM, et al. Perfluoropropane gas as a magnetic resonance lung imaging contrast agent in humans. Chest. 2013;144(4):1300–1310.
- Goralski JL, Chung SH, Glass TM, et al. Dynamic perfluorinated gas MRI reveals abnormal ventilation despite normal FEV1in cystic fibrosis. JCI Insight. 2020;5(2):e133400.
- Gutberlet M, Kaireit TF, Voskrebenzev A, et al. Free-breathing dynamic 19F gas MR imaging for mapping of regional lung ventilation in patients with COPD. Radiology. 2018;286(3):1040–1051.
- Neal MA, Pippard BJ, Hollingsworth KG, et al. Optimized and accelerated 19F-MRI of inhaled perfluoropropane to assess regional pulmonary ventilation. Magn Reson Med. 2019;82(4):1301-1311.
- Maunder, A, Rao, M, Robb, F, Wild, JM. Optimization of steady‐state free precession MRI for lung ventilation imaging with 19F C3F8 at 1.5T and 3T. Magn Reson Med. 2019; 81:1130–1142.
Figures
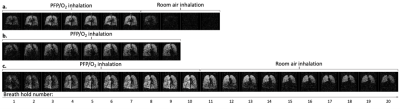
Figure 1: Multi-breath wash-in and wash-out of inhaled PFP/O2. A central coronal slice from a 3D 19F-MR image acquired during each breath hold is displayed. Images were acquired on alternate inhalations (ie. after 1, 3, 5, etc. inhalations of PFP/O2). PFP/O2 wash-in is visible over the initial breaths. The inhalation rig valve was then switched to deliver room air for visualisation of PFP/O2 gas wash-out. 1a. PFP/O2 wash-in and wash-out in a healthy volunteer. 1b. PFP/O2 wash-in in P1 (nb. wash-out images were not acquired in this participant). 1c. PFP/O2 wash-in and wash-out in P2.

Figure 2: %VV displayed against the PFP/O2 wash-in breath hold number (2a.) and the wash-out breath hold number (2b.). The measured %VV values, calculated from the overlay of the segmented 19F-MR image (ventilated lung volume) on the 1H anatomical image (total lung volume), are displayed as crosses (red: HV1, orange: P1, green: P2), with corresponding fitted exponential curves shown. The substantial change in patient %VV over consecutive wash-in breath holds demonstrates the potential insensitivity of conventional static imaging techniques at defining ventilated volume.
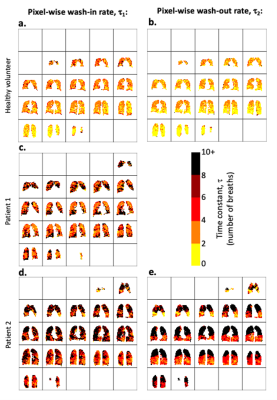
Figure 3: 3D voxel-wise exponential time constant estimation, where τ1 represents the number of wash-in respiratory cycles required to increase the 19F-MRI signal to 1 - 1/e (≈63.2%) of its calculated maximum value. τ2 represents the number of respiratory cycles required to reduce the 19F-MRI signal to 1/e (≈36.8%) of its calculated maximum value. Maps for wash-in τ1(3a., 3c., and 3d.) and wash-out τ2 (3b. and 3e.) are shown.

Figure 4: Histograms of global PFP/O2 wash-in τ1 (3a.) and wash-out τ2 (3b.) values. 3c. Median regional wash-in rates measured in eight lung sections. Pearson regression analysis of regional median τ1 values and participant FEV1 revealed a strong negative correlation (r = -0.79). An increased between-region spread of τ1 is visible. 3d. Median regional wash-out rates measured in eight lung sections. A strong negative correlation with FEV1 was measured (r = -0.79). Regression lines are displayed.