3218
Imaging the interplay of notch-DLL4 expression with pulmonary radiation injury using dynamic contrast enhanced ultrashort echo time imaging1Medical College of Wisconsin, Milwaukee, WI, United States
Synopsis
Lung injury after exposure to high-dose radiation during cancer treatment has been well described. Our goal in this study is to develop minimally invasive biomarkers for predicting radiation-induced lung injury and assess the role of DLL4 expression in response to radiation injury. We presented a non-invasive UTE-based DCE MRI method for in-vivo quantification of irradiation-induced vascular perfusion and permeability early changes in lungs using two rat models which only differ in 3rd chromosome and DLL4 expression on endothelium. Such knowledge is crucial for accurately evaluating the efficacy of radioprotectors and therapeutic agents, and for monitoring individuals with survivable radiation injury.
Introduction
Lung injury after exposure to high-dose radiation during cancer treatment has been well described. The injury to blood vessels is believed to be a primary determinant of the resultant effects of radiation in a variety of organs, including the lungs. Notch-DLL4 gene expression on endothelial cells is implicated in vascular remodeling and its inhibition can cause dysfunctional angiogenesis. Our goal in this study is to develop minimally invasive biomarkers for predicting radiation-induced lung injury and assess the role of DLL4 expression in the response to radiation injury. SS (DLL4-high) and SS.BN3 (DLL4-low) consomic rat strains differ in 3rd chromosome. The SS.BN3 congenic strain was obtained by introgressing the BN chromosome-3 into the parental Dahl-SS strain to localize the role of DLL4 which is coded on chromosome-3 and supported by prior studies on the differing levels of DLL4 expression in these strains. We report the use of high temporal resolution (~0.3s) DCE-MRI to characterize radiation-induced changes in organ permeability and perfusion using ultrashort echo time (UTE) pulse sequences. The contrast agent kinetics and radiation-induced changes in lungs in SS and SSBN3 rats were evaluated.Methods
A total 9 SS and 8 SS.BN3 rats (11-12 week females, average weight = 200 g) were randomized into four study groups, i.e., SS 0 Gy (n=4), SS 13 Gy (n=4), SSBN3 0 Gy (n=4), and SSBN3 13 Gy (n=4). The 13-Gy rats were exposed to a single dose of X-rays with one hind leg shielded (leg-out partial body irradiation; PBI) without anesthesia. Anesthetized rats were imaged, in a prone position, at 3- and 6-weeks post-irradiation on a 9.4T Bruker Biospec MRI scanner with 30-cm bore diameter and equipped with 4-element surface coil. The UTE imaging parameters were as follows: TR = 3.1ms, TE = 0.4ms, flip angle = 15°, matrix = 80x80, FOV = 40x40 mm2, slice thickness = 1.5mm, acquisition bandwidth = 1190 Hz/pixel, #averages = 1, radial acquisition with undersampling factor of 3, and dynamic acquisition of 1000 frames at ~350ms temporal resolution. After baseline imaging, 0.05 mL Gadovist contrast agent was injected at rate of 10 mL/min, followed by 0.6 mL saline flush through the tail vein using a power injector. The images were analyzed by first applying a motion correction filter to remove respiratory induced motion, and contrast agent kinetics was compared.Results
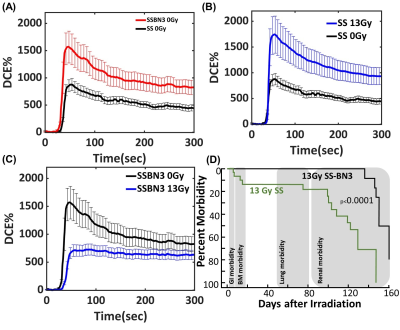
Vascular dysfunction in the lungs resulted in significantly altered contrast uptake and/or washout measured at 42 days after 13 Gy leg-out PBI. SS.BN3 rats express lower notch-DLL4 protein on blood vessels compared to age matched SS rats. The area under the contrast enhancement curve (IAUC) was found to be 104, 81, and 12% for SS 13Gy, SSBN3 0Gy, and SSBN3 13Gy rats compared SS 0Gy rats. Washout rate for SS 0Gy, SS 13Gy, SSBN3 0Gy, and SSBN3 13Gy rats are found to be 4.33, 7.2, 7.0, and 0.2 S-1 for IAUC (100). However, the impact of radiation injury on both rats was opposite and paradoxical. While SS rats have lower Gd uptake in lungs pre-radiation, (Fig.1A) they exhibited significant increase in Gd uptake and retention post radiation injury indicated increases in vascular permeability and/or vessel growth (Fig. 1B). SS.BN3 rats exhibited a strong decrease in contrast agent uptake and retention following radiation injury (Fig. 1C). These finding were intriguing as SS rats had significantly higher mortality and early onset of radiation injury compared to SS.BN3 rats (Fig.1D). This suggests that levels of DLL4 expression on endothelium drive the response to radiation injury by either affecting the vessel permeability or by affecting vessel regression in response to injury.Discussion and Conclusions
We presented a non-invasive UTE-based DCE MRI method showing the potential for in vivo quantification of irradiation-induced vascular perfusion and permeability early changes in lungs using two rat models which only differ in 3rd chromosome and DLL4 expression on endothelium. Such knowledge will be crucial for accurately evaluating the efficacy of radioprotectors and therapeutic agents, as well as for monitoring individuals with survivable radiation injury.Acknowledgements
Funding from Daniel M. Soref Charitable Trust, MCW, USA.References
1. Fish et al, Health Phys. 111:410-419
2. Ghosh et al, Int J Rad Onc Biol Phys.74:192-199
3. Medhora et al, J Nucl Med. 57:1296-1301
4. Flister et. al., Breast Cancer Res Treat. 165(1):53-64.
Figures