3197
DCE-MRI for the Assessment of Outcome of CyberKnife Stereotactic Radiosurgery for Patients with Spinal Metastases1Peking University Third Hospital, Beijing, China, 2Peking University International Hospital, Beijing, China
Synopsis
The imaging methods used to evaluate the efficacy of radiosurgery in spinal metastases have certain limitations. DCE-MRI can help determine the vascularity and hemodynamics of tumors in vivo, and can be used to evaluate local tumor response. Twenty-seven patients with 39 lesions were included. Post-treatment Kep, ΔKtrans and ΔKep in the non-progressive disease group were significantly lower than the corresponding values in progressive disease group. Post-treatment Ve and ΔVe in the non-progressive disease group were significantly higher than that of the progressive disease group. ΔKtrans had the highest diagnostic efficiency, with an AUC of 0.821.
Introduction
Spinal metastases can cause vertebral compression fractures, compress nerve roots and the spinal cord, cause severe pain and neurological complications, and have a tremendous impact on patients' quality of life. Unlike traditional palliative radiotherapy, CyberKnife radiosurgery can deliver large doses of precise irradiation to spinal metastases while minimizing dose to the surrounding sensitive tissues such as the spinal cord, allowing local control of the lesion and improving the stability of the spine [1]. At present, the evaluation of imaging efficacy mainly depends on the morphological changes of the tumor before and after treatment, and conventional MRI is the most commonly used imaging evaluation method. The increase in lesion volume is usually defined as progression. However, the size of spinal tumors is not easy to measure, and some tumors do not change significantly when assessed using traditional imaging methods. Hwang et al. [2] showed that 49.4% of spinal metastases remained unchanged in tumor volume after stereotactic radiosurgery when imaged with conventional MRI. DCE-MRI can help determine the vascularity and hemodynamics of tumors in vivo in a non-invasive manner [3], and can be used to explore the survival status of spinal metastatic tumor cells after treatment to evaluate local tumor response.Methods
We enrolled patients with spinal metastases who were treated with CyberKnife radiosurgery. The first follow-up was performed at 3 months after CyberKnife radiosurgery. The first follow-up included conventional MRI and DCE-MRI scans, followed by a 3-month cycle of follow-up examinations; MRI or CT examinations were performed according to clinical needs. The imaging efficacy evaluation was based on the RECIST version 1.1 [4], and the lesions were divided into progressive disease (PD) group and non-PD group according to the evaluation results. The hemodynamic parameters (volume transfer constant [Ktrans], rate constant [Kep], and extravascular space [Ve]) before and after treatment between the groups were analyzed. Area under the curve (AUC) values were calculated.Results
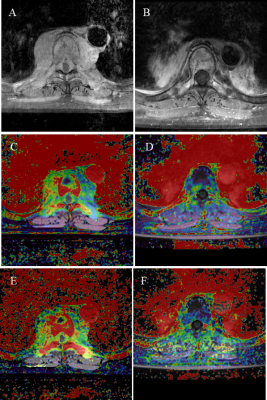
A total of 27 patients with 39 independent spinal lesions were included. Eighteen patients had a single lesion each, while nine patients had multiple lesions. The median follow-up time was 18.6 months (6.2-36.4), with a mean of 18.5 months. There were 27 lesions in the non-PD group and 12 lesions in the PD group. Post-treatment Kep, ΔKtrans and ΔKep in the non-PD group (0.959 min-1, -32.6% and -41.1%, respectively) were significantly lower than the corresponding values in PD group (1.429 min-1, 20.4% and -6.0%; p < 0.05). Post-treatment Ve and ΔVe (0.223 and 27.8%, respectively) in the non-PD group were significantly higher than that of the PD group (1.429 min-1, 0.165 and -13.5%, p < 0.05). The representative lesions in the non-PD group and the PD group are shown in Figure 1 and Figure 2. Among all parameters, ΔKtrans showed the highest diagnostic efficiency, with an AUC of 0.821.Discussion
Early predicting and assessing local tumor response are very important for the treatment of patients. A robust radiation dose treatment regime to recurrent tumor is essential for successful salvage, because recurrent tumor is likely to cause acquired radiation resistance as tumors develop an adaptive response and epigenetic changes [1]. Stereotactic radiosurgery can destroy tumor blood vessels and inhibit neovascularization, which leads to the destruction of the tumor internal microenvironment [5]. DCE-MRI parameters can sensitively detect changes in the blood flow state of the lesion tissue before and after treatment, and reflect the curative effect on the basis of the magnitude of the parameter changes before and after treatment. In our study, Ktrans, Kep and Ve were found to be of great value in evaluating the efficacy of CyberKnife radiosurgery in spinal metastases. Ktrans and Kep values are closely related to the state of tumor microcirculation and angiogenesis. In comparison with normal blood vessels, tumor blood vessels have higher permeability and perfusion, which means that tumor tissues have higher Ktrans and Kep values [6]. Post-treatment Kep, ΔKtrans, and ΔKep in the non-PD group were significantly lower than those in the PD group. This might be due to the damage to the vascular structure of tumor tissues by radiation therapy, which inhibited the regeneration of tumor blood vessels, leading to reductions in post-treatment Ktrans, post-treatment Kep, ΔKtrans, ΔKep. In contrast, tumors in the PD group were resistant to radiotherapy, resulting in less damage to the structure and function of tumor vascular tissues and more active tumor vascular regeneration, which ensured a less obvious parameter decline, even increase. Post-treatment Ve and ΔVe in the non-PD group were significantly higher than the PD group. Ve represented the volume of extravascular extracellular space (EES) per unit volume of tissue. Tumor tissue blood vessels were destroyed after radiotherapy, and the intravascular space volume would decrease, so the EES volume and its proportion would increase, resulting in an increase in Ve [7].Conclusion:
DCE-MRI, as the best non-invasive examination method for reflecting tissue hemodynamic information, has high accuracy in determining the early efficacy of CyberKnife radiosurgery. It can compensate for the limitations of conventional MRI in the evaluation of therapeutic efficacy, especially when the change in tumor morphology is not obvious after treatment. This provides valuable information for future clinical practice and guides doctors in planning treatment options.Acknowledgements
This study was supported in part by the National Natural Science Foundation of China (81971578, 81701648), and the Key Clinical Projects of the Peking University Third Hospital (BYSY2018007).References
1. Katsoulakis E, Kumar K, Laufer I, Yamada Y. Stereotactic Body Radiotherapy in the Treatment of Spinal Metastases. Seminars in radiation oncology. 2017; 27: 209-17.
2. Hwang YJ, Sohn MJ, Lee BH, Kim SY, Seo JW, Han YH, et al. Radiosurgery for metastatic spinal tumors: follow-up MR findings. AJNR American journal of neuroradiology. 2012; 33: 382-7.
3. Sujlana P, Skrok J, Fayad LM. Review of dynamic contrast-enhanced MRI: Technical aspects and applications in the musculoskeletal system. Journal of magnetic resonance imaging : JMRI. 2018; 47: 875-90.
4. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European journal of cancer (Oxford, England : 1990). 2009; 45: 228-47.
5. Lindblom EK, Hui S, Brooks J, Dasu A, Kujawski M, Toma-Dasu I. Radiation-induced Vascular Damage and the Impact on the Treatment Outcome of Stereotactic Body Radiotherapy. Anticancer research. 2019; 39: 2721-7.
6. Sun NN, Liu C, Ge XL, Wang J. Dynamic contrast-enhanced MRI for advanced esophageal cancer response assessment after concurrent chemoradiotherapy. Diagnostic and interventional radiology (Ankara, Turkey). 2018; 24: 195-202.
7. Mamata H, Tokuda J, Gill RR, Padera RF, Lenkinski RE, Sugarbaker DJ, et al. Clinical application of pharmacokinetic analysis as a biomarker of solitary pulmonary nodules: dynamic contrast-enhanced MR imaging. Magnetic resonance in medicine. 2012; 68: 1614-22.
Figures