3195
High-Contrast Lumbar Spinal Bone Imaging Using a 3D Slab-Selective UTE Sequence1Department of Radiology, University of California, San Diego, San Diego, CA, United States, 2GE HealthCare, San Diego, CA, United States, 3Radiology Service, Veterans Affairs, San Diego Healthcare System, San Diego, CA, United States
Synopsis
To explore the application of a 3D slab-selective UTE sequence as a superior technique to 3D ZTE at 3T field strength and to compare with 3D CT as a gold standard in Lumbar spinal bone assessment.
Introduction
Computed tomography (CT) offers both high-resolution and high-contrast imaging of bone, and is recognized as the gold standard imaging modality for spinal bone evaluation. However, due to the ionizing radiation exposure associated with CT, this imaging modality is not recommended for children or for patients requiring frequent examinations. In comparison, magnetic resonance imaging (MRI) is a superior method given that it does not involve radiation exposure and that it is able to provide high-contrast visualization of soft tissue. However, bone signals cannot be effectively detected by conventional MRI sequences due to their low proton density and short transverse relaxation times of bone (T2~390 μs at 3T). Fortunately, ultrashort echo time (UTE) and zero echo time (ZTE) sequences with echo times (TEs) less than 100 μs are able to capture these bone signals1,2. Most recently, ZTE sequences combined with data post-processing have been applied to skull, shoulder, cervical spine, and hip imaging, and have demonstrated similar contrast to CT3-5. However, ZTE application in the lumbar spine may encounter some technical challenges, including artifacts caused by respiratory motion resulting from the use of non-selective rectangular radiofrequency (RF) pulses, and spatial signal inhomogeneity caused by the potential coil ring-down factor. In contrast, UTE takes advantage of slab-selectivity using a soft pulse for signal excitation that can reduce artifacts related to respiratory motion. Further, because UTE allows for adjustment of echo time, the tissue signal inhomogeneity induced by the coil ring-down factor can be reduced. In this study, we investigated the performance of 3D UTE and ZTE sequences in lumbar spinal bone imaging and compared UTE imaging with the gold standard CT imaging.Methods and Materials
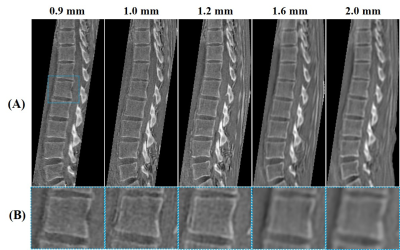
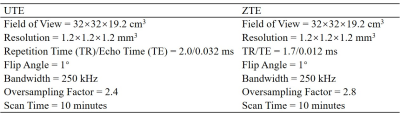
Figure 1 shows the features of 3D ZTE and UTE sequences. The ZTE sequence utilizes a non-selective rectangular RF pulse with short duration for excitation (8 μs), followed by 3D center-out radial sampling (Fig. 1A). To minimize echo time, readout gradients are turned on prior to signal excitation so that the gradient encoding begins simultaneously with signal excitation. 3D UTE sequence enables slab selection by using a soft-half pulse for excitation (~1 ms) together with a slice-selective gradient (Fig. 1B). After excitation, the spatial-encoding gradient is turned on and simultaneous data acquisition begins. In data post-processing, the N4ITK bias correction algorithm was performed for both UTE and ZTE images. Then, the corrected images were logarithmically transformed and inverted to generate high bone contrast.In this study, we first to compare 3D UTE and ZTE sequences in lumbar spinal bone imaging, a 29-year-old healthy female volunteer was scanned. All scans were performed in coronal plane to exclude soft tissue in the abdomen (i.e., the major tissues to cause respiratory motion artifacts). The detailed sequence parameters of UTE and ZTE are found in Table 1. Second, to investigate the best resolution for UTE spinal bone imaging, five scans with different isotropic resolutions (i.e., 0.9, 1.0, 1.2, 1.6, and 2 mm3) were performed on the above-described healthy volunteer. A 72-year-old male patient with spinal bone fractures was also recruited and underwent UTE bone imaging for comparison against his most recent CT images.
Results and Discussion
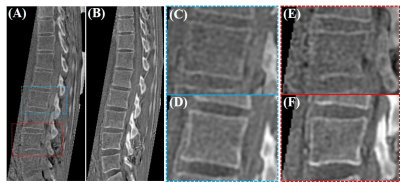
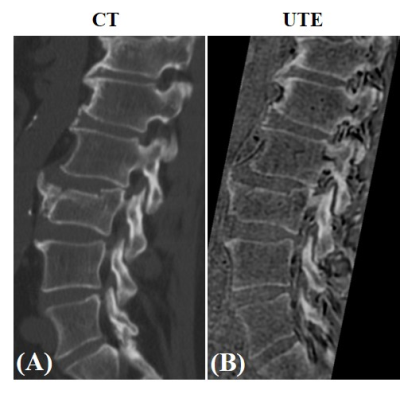
Figure 2 presents the comparative ZTE and UTE lumbar spinal bone images of the healthy volunteer. As expected, UTE imaging showed much better bone structural information compared with ZTE. Figure 3 presents the five UTE scans with different resolutions for this same volunteer. The signal-to-noise ratio (SNR) was lower when the resolution was higher. However, when the resolution was too low, the bone structures were not very well-demonstrated. The resolution with a voxel size of 1.2 mm3 showed a good balance between image SNR and bone structures. Figure 4 compares UTE and CT spine bone images of the patient with spinal bone fractures. Vertebral fracture was clearly visible in both CT and UTE images. There were also high levels of agreement between UTE MRI and gold standard CT scan.Conclusion
The 3D UTE sequence was superior to the 3D ZTE sequence in lumbar spinal bone imaging because of its reduced sensitivity to respiratory motion. Furthermore, the UTE imaging of spine offered comparable results to gold standard CT. Therefore, 3D UTE MRI is suitable as a clinically-compatible technique in spinal bone imaging and has strong potential for effective incorporation into the clinical workflow.Acknowledgements
The authors acknowledge grant support from the NIH (R01NS092650, and R21AR075851), Veterans Affairs (I01RX002604 and I01CX001388), and GE Healthcare.References
1. Du J, Carl M, Bydder M, Takahashi A, Chung CB, Bydder GM. Qualitative and quantitative ultrashort echo time (UTE) imaging of cortical bone. J Magn Reson. 2010 Dec;207(2):304–11.
2. Delso G, Wiesinger F, Sacolick LI, Kaushik SS, Shanbhag DD, Martin H. Clinical Evaluation of Zero-Echo-Time MR Imaging for the Segmentation of the Skull. 2014;417–23.
3. Breighner RE, Bogner EA, Lee SC, Koff MF, Potter HG. Evaluation of Osseous Morphology of the Hip Using Zero Echo Time Magnetic Resonance Imaging. Am J Sports Med [Internet]. 2019 Oct 21;47(14):3460–8. Available from: https://doi.org/10.1177/0363546519878170
4. Wiesinger F, Sacolick LI, Menini A, Kaushik SS, Ahn S, Veit-Haibach P, et al. Zero TE MR bone imaging in the head. Magn Reson Med. 2016 Jan;75(1):107–14.
5. Weiger M, Brunner DO, Dietrich BE, Pruessmann KP, Colin FM. ZTE Imaging in Humans. 2013;332:328–32.
Figures
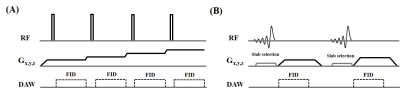
Figure 1. 3D ZTE (A) and UTE (B) sequences. The ZTE sequence utilizes a non-selective rectangular RF pulse with short duration for excitation, followed by 3D center-out radial sampling. To minimize echo time, readout gradients are turned on prior to signal excitation so that the gradient encoding can begin simultaneously with signal excitation. The 3D UTE sequence enables slab selection by using a soft-half pulse for excitation together with a slice-selective gradient. After excitation, the spatial encoding gradient is turned on and simultaneous data acquisition begins.