3190
Spinal Giant Cell Tumor of Bone: Immunohistochemistry and Preoperative Magnetic Resonance Imaging Features for Prognostic Prediction1Radiology, Peking University Third Hospital, Beijing, China, 2Center for Functional Onco-Imaging, Irvine, CA, United States, 3Radiology, Peking University International Hospital, Beijing, China
Synopsis
A new evaluating system based on tumor immunohistochemistry and preoperative MRI features in spinal giant cell tumor of bone to predicting overall survival of total en bloc spondylectomy patients with over 2 years follow up. The largest lesion diameter (>4.2 cm) and the vertebral compression were independent predictors of postoperative recurrence. According to Kaplan-Meier survival analysis, the cystic change in the lesion and the degree of compression ≥50% suggest a worse clinical outcome. The expression levels of vascular endothelial growth factor and p53 gene have no obvious clinical significance on the survival outcome. H3F3A was positively expressed in our cohort.
Objectives
Giant cell tumor of bone (GCTB) is classified as an intermediate tumor with a local recurrent rate of 20–50%. The recurrence rate of GCTB that occurs in the spine is higher than the average. To date, how to identify malignant GCTB as early as possible is the key and difficult point in the diagnosis and treatment process.In this study, patients diagnosed with spinal GCTB by pathology after total en bloc spondylectomy (TES) were reviewed and analyzed to explore the predictive value of preoperative magnetic resonance imaging (MRI) characteristics for postoperative recurrence. At the same time, VEGF (vascular endothelial growth factor), p53 (p53 tumor suppressor gene) and H3F3A (H3.3 G34W) are used to determine the correlation with prognosis through immunohistochemical analysis.
Methods
Demographic and clinic pathological characteristics of spinal GCTB patients afterTES surgery with diagnosed from March 2010 to February 2018 were identified and reviewed. Univariate and multivariate logistic regression analyses were carried out to identify the independent characteristics, and Keplan-Meier survival curves were generated for comparative visualization.All postoperative paraffin specimens of all patients were obtained and HE (Hematoxylin and Eosin) staining was performed to reconfirm the diagnosis and then immunohistochemical analysis. The expression levels of VEGF, p53 and H3F3A were independently evaluated using a classic scoring system. The correlation between the expression levels of the three biomarkers and the clinical outcome of the patients was analyzed. P≤0.05 is considered statistically different.
Results
Of the 62 patients, 17 had recurrence and 45 did not. The recurrence rate was 27.4%. Univariate analysis showed that there was no significant difference in age, gender, treatment, multi-vertebral body involvement, location, boundary, expansile mass, paravertebral soft tissue mass and MRI signal. The logistic regression analysis showed that there were two significant recurrence predictors found in our study: vertebral compression and largest lesion diameter (>4.2 cm). The sensitivity and specificity for distinguishing the recurrence and non-recurrence of GCTB were 94.1% and 42.2%, respectively, and the AUC was 0.70. Through survival analysis, we found that patients with vertebral compression ≥50% and multiple cystic changes in the lesion have a worse prognosis (P<0.05). H3F3A was positively expressed in all patients in the cohort. VEGF is divided into low expression (24/62) and high expression (38/42), p53 is divided into negative (42/62) and positive (20/62). Through multivariate logistics regression analysis, no correlation with postoperative recurrence was found.Conclusions
For patients with spinal GCTB, preoperative MRI showed cystic change in the lesion and vertebral compression ≥50%, suggesting a poor prognosis. The maximum diameter of the lesion greater than 4.2cm may indicate an increased possibility of postoperative recurrence. H3F3A is often positive in benign and malignant spinal GCTB, and the expression of VEGF and p53 may not be associated with postoperative recurrence.Acknowledgements
This study was supported in part by the National Natural Science Foundation of China (81971578, 81701648), and the Key Clinical Projects of the Peking University Third Hospital (BYSY2018007).
References
1. He YF, Zhou Y, Zhang J, Yuan F, Wang J, Du LJ, et al. Tumor immunohistochemistry and preoperative magnetic resonance imaging features predict local recurrence of giant cell tumor of bone following intralesional curettage. Oncol Lett. 2019;17(2):1425-34.
2. Yokogawa N, Murakami H, Demura S, Kato S, Yoshioka K, Shimizu T, et al. Total spondylectomy for Enneking stage III giant cell tumor of the mobile spine. Eur Spine J. 2018;27(12):3084-91.
3. Jia Q, Chen GH, Cao JS, Yang XH, Zhou ZH, Wei HF, et al. Clinical features and prognostic factors of pediatric spine giant cell tumors: report of 31 clinical cases in a single center. Spine J. 2019;19(7):1232-41.
4. Ouyang HQ, Jiang L, Liu XG, Wei F, Yang SM, Meng N, et al. Recurrence Factors in Giant Cell Tumors of the Spine. Chinese Med J-Peking. 2017;130(13):1557-63.
5. Yin HB, Yang XH, Xu W, Li BB, Li B, Wang T, et al. Treatment and outcome of primary aggressive giant cell tumor in the spine. Eur Spine J. 2015;24(8):1747-53. 22. Si MJ, Wang CG, Wang CS, Du LJ, Ding XY, Zhang WB, et al. Giant cell tumours of the mobile spine: characteristic imaging features and differential diagnosis. Radiol Med. 2014;119(9):681-93.
6. Shi LS, Li YQ, Wu WJ, Zhang ZK, Gao F, Latif M. Imaging appearance of giant cell tumour of the spine above the sacrum. Brit J Radiol. 2015;88(1051).
7. Goldenberg RR, Campbell CJ, Bonfiglio M. Giant-cell tumor of bone. An analysis of two hundred and eighteen cases. J Bone Joint Surg Am. 1970;52(4):619-64.
8. Siebenrock KA, Unni KK, Rock MG. Giant-cell tumour of bone metastasising to the lungs. A long-term follow-up. J Bone Joint Surg Br. 1998;80(1):43-7.
9. Kremen TJ, Jr., Bernthal NM, Eckardt MA, Eckardt JJ. Giant cell tumor of bone: are we stratifying results appropriately? Clin Orthop Relat Res. 2012;470(3):677-83.
10. Wang H, Wan N, Hu Y. Giant cell tumour of bone: a new evaluating system is necessary. Int Orthop. 2012;36(12):2521-7. 11. Quattrini I, Pollino S, Pazzaglia L, Conti A, Novello C, Ferrari C, et al. Prognostic Role of Nuclear Factor/IB and Bone Remodeling Proteins in Metastatic Giant Cell Tumor of Bone: A Retrospective Study. J Orthop Res. 2015;33(8):1205-11.
12. Hatano Y, Nakahama K, Isobe M, Morita I. Tumor associated osteoclast-like giant cells promote tumor growth and lymphangiogenesis by secreting vascular endothelial growth factor-C. Biochem Bioph Res Co. 2014;446(1):149-54.
13. Yalcinkaya U, Ugras N, Kabul S, Ocakoglu G, Bilgen MS. Prognostic Value of P53 Protein Expression in Giant Cell Tumor of Bone. Pol J Pathol. 2015;66(4):389-96.
14. Okubo T, Saito T, Mitomi H, Takagi T, Torigoe T, Suehara Y, et al. p53 mutations may be involved in malignant transformation of giant cell tumor of bone through interaction with GPX1. Virchows Archiv. 2013;463(1):67-77.
Figures
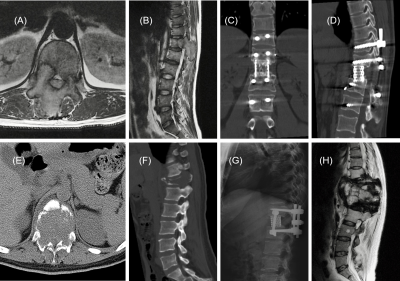
Fig.2. Top panel: A 35-year-old man,(A) and (B): MR images showed a mass on the T12 vertebra, bilateral pedicle and lamina with extension into the spinal canal, managed with en bloc resection (C), at a 36-month follow-up review, there was no evidence of recurrence (D), and now the patient is still on visit.
Bottom panel: A 35-year-old woman, maximum diameter of lesion is 55mm (E),with pathologic fracture of the T12 vertebra (F) , managed with en bloc resection (G). The sagittal T2-WI MR image at 12-month follow-up, recurrence was detected (H), and confirmed by pathology with puncture.
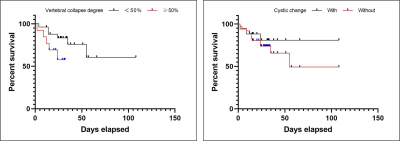
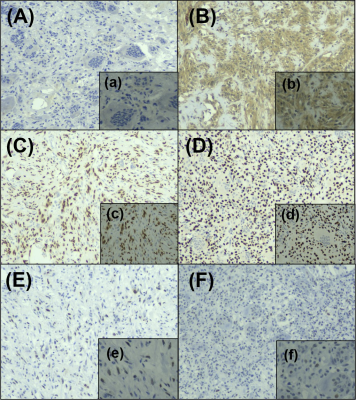
Fig.4. (A) IHC grade 0, virtually no VEGF immunoreactivity. (B) IHC grade 3, diffuse strong immunoreactivity, VEGF expression is seen in approximately 80 % of tumor cells in a case of conventional GCTB. (C) -(D) scattered positive cells for H3F3A are observed. (E) scattered positive cells (more than 10 %) for p53 are observed throughout the primary lesion in these two cases. (F) absence of p53 expression in almost all tumor cells.
Magnification: A, B, C, D, E, F= 10×; a, b, c, d, e, f= 20×. IHC, immunohistochemistry.