3185
Clinical feasibility of ultrafast lumbar spine magnetic resonance imaging: a preliminary report1Osaka University Graduate School of Mediine, Suita, Japan, 2Canon Medical Systems Corporation, Otawara, Japan, 3Department of Radiology, Yukoukai General Hospital, Ibaraki, Japan
Synopsis
Using deep learning reconstruction, we developed an ultrafast lumbar MRI protocol with a total acquisition time of 1 minute 53 seconds. Compared with the standard MRI protocol, the interprotocol agreement for the classification of degenerative lumbar changes was almost perfect or substantial agreement with weighted kappa values of 0.72 to 0.84. The two protocols showed 100% concordance for the detection of clinically significant pathologies. Our preliminary study demonstrated that the ultrafast lumbar MRI protocol adequately preserved diagnostic equivalency with the standard protocol, suggesting that it could be an alternative protocol for restless patients and in an emergency setting.
Background
Magnetic resonance imaging (MRI) is arguably the most powerful imaging modality for spinal disorders because of the variety of contrast mechanisms and that it requires no ionizing radiation. However, its relatively long acquisition time limits its clinical use in uncooperative patients and time-critical disease. The recent wide availability of a machine learning algorithm has led to development of several deep learning-based methods for improving the quality of brain MRI.1-5 However, the application of deep learning-based methods to shorten the acquisition time of spinal MRI has not been reported. In the current study, we developed an ultrafast lumbar MRI protocol with the total acquisition time of 1 minute 53 seconds using a combination of existing acceleration techniques and the recently commercialized deep-learning reconstruction tool (AiCE; Advanced Intelligent Clear-IQ Engine, Canon Medical Systems)5 to address the abovementioned issue.
Purpose
The purpose of this study was to compare the diagnostic performance of our ultrafast lumbar MRI protocol and a standard lumbar MRI protocol for the classification of lumbar degenerative changes and the detection of clinically significant lumbar pathologies.
Materials and methods
Following approval by the institutional review board, this prospective study included 38 consecutive patients with clinical indications for MRI of the lumbar spine. Using a 1.5 T MRI system (Vantage Orian 1.5 T, Canon Medical Systems, Tochigi, Japan), all the patients underwent both the standard protocol that included five sequences (sagittal T1-weighted, T2-weighted, and short-TI inversion recovery [STIR] sequences, axial T2-weighted sequence) and the ultrafast protocol including the equivalent five sequences (Figure 1). The total scan time for the standard protocol was 12 minutes 31 seconds and that for the ultrafast protocol was shortened to 1 minute 53 seconds by using a reduced number of excitations, a lower oversampling rate, a higher acceleration factor in compressing sensing, and a lower spatial resolution. The deep-learning reconstruction was applied to the ultrafast protocol to improve the degenerated image quality. The standard and ultrafast images from each patient were reviewed to grade lumbar degenerative changes and for detection of clinically significant pathologies. The degenerative changes included central canal stenosis graded according to Guen et al.,6 foraminal stenosis graded according to Lee et al.,7 Modic endplate changes,8 and lumbar disc pathologies assessed by the Combined Task Force and Rijin classification.9 Clinically significant pathologies were defined as severe lumbar canal stenosis, severe foraminal stenosis, disc extrusion or sequestration, acute vertebral compression fracture, vertebral osteonecrosis, or neoplasm of the spinal canal or vertebral column.
Results
The interprotocol agreement regarding the evaluation of degenerative changes was classified as almost perfect or substantial agreement with a weighted kappa value of 0.72 for central canal stenosis, 0.82 for foraminal stenosis, 0.84 for endplate change, and 0.78 for disc pathologies. We detected 25 clinically significant pathologies, which included eight severe lumbar canal stenoses, seven severe foraminal stenoses, four disc extrusions, two acute vertebral fractures, two vertebral osteonecroses, and two vertebral tumors. The concordance rate of the two protocols for the detection of clinically significant pathologies was 100% (Figure 2 and 3).
Discussion
Despite the trade-off relationship between image quality and acquisition time on MRI, the proposed ultrafast lumbar MRI protocol preserved overall diagnostic ability. This preliminary result seems to imply that the deep-learning reconstruction compensates for image degradation caused by reducing the acquisition time. Another possible implication is that the loss of image quality precedes diagnostic ability.10
Conclusion
The diagnostic performance of our proposed ultrafast lumbar MRI protocol showed a high concordance rate with that of the standard protocol.
Acknowledgements
No acknowledgement found.References
1. Jiang D, Dou W, Vosters L, et al. Denoising of 3D magnetic resonance images with multi-channel residual learning of convolutional neural network. Jpn J Radiol 2018;36(9):566–574.
2. Wang H, Zheng R, Dai F, et al. High-field MR diffusion-weighted image denoising using a joint denoising convolutional neural network. J Magn Reson Imaging 2019;50(6):1937–1947.
3. Xie D, Li Y, Yang H, Bai L, et al. Denoising arterial spin labeling perfusion MRI with deep machine learning. Magn Reson Imaging 2020;68:95–105.
4. Kim KH, Choi SH, Park SH. Improving Arterial Spin Labeling by Using Deep Learning. Radiology 2018;287(2):658-666.
5. Kidoh M, Shinoda K, Kitajima M, et al. Deep learning based noise reduction for brain MR imaging: tests on phantoms and healthy volunteers. Magn Reson Med Sci. 2020 Aug 3;19(3):195-206.
6. Lee GY, Lee JW, Choi HS, Oh KJ, et al. new grading system of lumbar central canal stenosis on MRI: an easy and reliable method. Skeletal Radiol. 2011;40(8):1033-1039.
7. Lee S, Lee JW, Yeom JS, et al. A practical MRI grading system for lumbar foraminal stenosis. AJR Am J Roentgenol. 2010;194(4):1095-1098.
8. Modic MT, Ross JS. Lumbar degenerative disk disease. Radiology 2007;245(1):43-61.
9. Fardon DF, Milette PC. Combined Task Forces of the North American Spine Society, American Society of Spine Radiology, and American Society of Neuroradiology. Spine (Phila Pa 1976). 2001 Mar 1;26(5):E93-E113.
10. Terae S, Miyasaka K, Kudoh K, et al. Wavelet compression on detection of brain lesions with magnetic resonance imaging. J Digit Imaging. 2000;13(4):178-190.
Figures
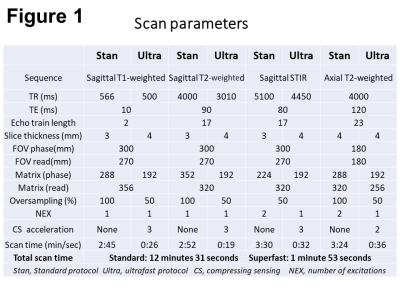
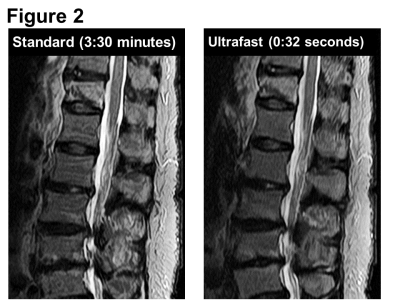
Figure 2. A 67-year-old woman with severe back pain.
Standard sagittal STIR image (scan time, 3:30 minutes) shows diffuse hyperintensity and a low-intensity line in the 12th thoracic vertebra, suggesting an acute compression fracture. The ultrafast STIR image (0:32 seconds) shows the equivalent lesion almost as clearly.
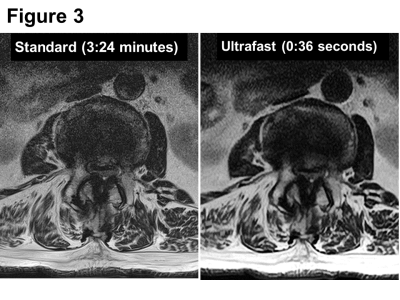
Figure 3. A 59-year-old man with intermittent claudication.
On axial T2-weighted images, both standard (3:24 minutes) and ultrafast (0:36 seconds) sequences comparably demonstrate severe central canal stenoses.