3179
Feasibility Study of 31P Multivoxel Spectroscopy for the detection of Alkaline Inorganic Phosphate in multiple compartments of the lower leg at 3T1Human Magnetic Resonance Center, Institute for Applied Life Sciences, University of Massachusetts Amherst, Amherst, MA, United States, 2Kinesiology, University of Massachusetts Amherst, Amherst, MA, United States
Synopsis
In this study, we demonstrate the feasibility and sensitivity of 31P two-dimensional CSI sequence for the detection of alkaline Pi in the soleus, tibialis anterior, and gastrocnemius muscles in six healthy volunteers in rest. The major finding of the current study was the consistent detection of an alkaline Pi from the cytosolic Pi signal in the volume localized 31P MRS from the resting muscle in healthy human subjects at 3T using decoupling technique. This study provides the proof of concept for a non-invasive and localized method to determine the alkaline Pi, a potential index of mitochondrial density at rest.
Introduction
Magnetic resonance spectroscopy is unmatched in its strength to measure tissue biochemistry in intact humans without the need for invasive methods. In particular, it has been used extensively to monitor 31-phosphorus (31P) metabolites in skeletal muscle to provide an in vivo measure of metabolic fluxes and mitochondrial function. For the past few decades, due to the large volume and easy accessibility of the skeletal muscles of the human leg, 31P-MRS has been applied to detect and quantify phosphorous metabolites using surface coils. This approach takes advantage of the rapid drop-off in signal with increasing distance from the coil to localize the signal to superficial muscles1. However, such approach cannot distinguish the metabolic properties of different muscle groups. Recently, several pieces of evidence suggests that the presence of an alkaline inorganic phosphate (Pi2) peak in the skeletal muscle may be related to mitochondrial content2,3, a key factor regulating energy metabolism. In this context, metabolic mapping strategies to examine specific muscle groups are very attractive to provide spatial information on the concentration of Pi2 and therefore, perhaps, mitochondrial content. In the present study, we performed 31P localized two-dimensional (2D) MRSI using a birdcage coil to detect and quantify with spatial specificity of the alkaline Pi resonance in the soleus, gastrocnemius and tibialis anterior muscles at 3T.Materials and Methods
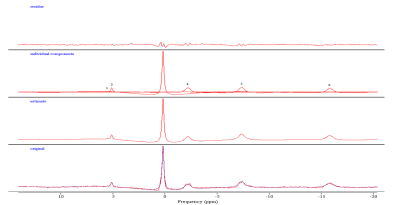
Six healthy volunteers (32 ± 8 yrs) participated in this study. 2D-MRSI was performed using a Siemens Skyra scanner. 2D-MRSI data were acquired with a birdcage quadrature transmit receive proton (1H) and phosphorous (31P) dual tuned knee coil (length: 35cm, diameter: 15cm) positioned around the lower leg. A two-dimensional (2D) pulse acquired 31P MRSI was used with the following parameter: TR/ TE: 2000ms / 2.3 ms; FOV: 160x 160 mm; Matrix: 8x8; vector size: 2048; Thickness: 80 mm; Average: 64; FA: 900 ; Bandwidth: 4000Hz; Encoding: Weighted; Decoupling pulse: Waltz-4 with decoupling total duration of 40%. All measurements were performed within SAR limits. Magnetic field homogeneity was optimized on water using the 1H coil and confirmed on the PCr peak of the 31P signal to yield full width at half maximum (FWHM) of ~17 Hz. The carrier frequency was placed between the PCr and Pi peak. All the metabolites in the selected soleus, gastrocnemius and tibialis anterior were processed using jMRUI and quantified using AMARES non-linear least squares algorithm.Results
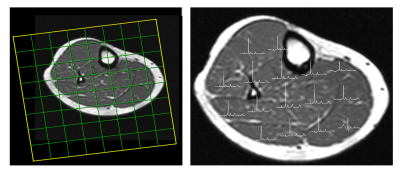
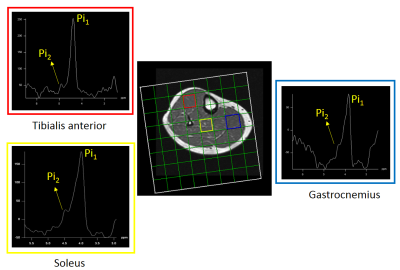
All the phosphorous metabolites were detected in the soleus, gastrocnemius and tibialis anterior of the lower leg. In addition, the separation between Pi1 and Pi2 was approximately 0.39 ppm was detected significantly in all three locations. Fig 1 shows a 36 years old healthy volunteer 2D CSI voxel location and multivoxel spectra on top of T1W MRI. Fig 2 shows the 36 years old healthy volunteer 2D CSI voxel location and selected single voxel spectrum and zoomed alkaline and cytosolic Pi regions from the tibialis anterior (red), soleus (yellow) and gastrocnemius (blue) muscles. Fig 3 shows the bar graph of the ratio of Pi2/Pi1 and Pi2/ B-ATP. Fig 4 shows the 36 years old healthy volunteer jMRUI fitting from the tibialis anterior. The concentration of Pi2 was not significantly different between muscles (0.36 ± 0.28 mM in the gastrocnemius, 0.28 ± 0.24 mM in the soleus, and 0.29 ± 0.47 mM in the tibialis anterior; P>0.05). Also, the Pi2 of the soleus muscle was significantly correlated to the tibialis anterior muscle (r = 0.94, P<0.05) whereas the other correlations did not reach significance (gastrocnemius and soleus: r = 0.62, P>0.05; gastrocnemius and tibialis anterior: r = 0.58, P>0.05).Discussion and Conclusions
This study demonstrates the feasibility of mapping and quantifying at 3T a small alkaline Pi peak from different skeletal muscles in the lower leg. Given the small chemical shift difference between the cytosolic and the alkaline Pi peaks, localized measurements had only been performed at 7T which affords higher spectral resolution and SNR2, 4, whereas attempt at 3T had been limited to coil-localized experiments5,6. We did not detect any significant differences in [Pi2] between the three muscles investigated, which is at odd with their previously documented contrasting fiber type. However, [Pi2] was significantly correlated between the soleus and tibialis anterior muscle, which is consistent with their antagonistic dorsi-/plantar-flexion roles and therefore similar metabolic constraints. Given the suggested link between Pi2 and mitochondrial content, the present findings provides the methodological basis to map mitochondrial content in multiple muscles and identify abnormalities in people suffering of mitochondrial myopathies or spatially defined muscle dysfunction (e.g. peripheral artery disease) in more broadly available 3T scanners.Acknowledgements
No acknowledgement found.References
1. Ackerman JJ, Grove TH, Wong GG, et al. Mapping of metabolites in whole animals by 31P NMR using surface coils. Nature. 1980;283(5743):167-70.
2. Kan HE, Klomp DW, Wong CS, et al. In vivo 31P MRS detection of an alkaline inorganic phosphate pool with short T1 in human resting skeletal muscle. NMR in biomedicine. 2010;23(8):995-1000.
3. Valkovic L, Chmelik M, Ukropcova B, et al. Skeletal muscle alkaline Pi pool is decreased in overweight-to-obese sedentary subjects and relates to mitochondrial capacity and phosphodiester content. Scientific reports. 2016;6:20087.
4. van Oorschot JW, Schmitz JP, Webb A, et al. 31P MR spectroscopy and computational modeling identify a direct relation between Pi content of an alkaline compartment in resting muscle and phosphocreatine resynthesis kinetics in active muscle in humans. PloS one. 2013;8(9):e76628.
5. Sedivy P, Dezortova M, Drobny M, et al. Origin of the (31) P MR signal at 5.3 ppm in patients with critical limb ischemia. NMR in biomedicine. 2020;33(6):e4295.
6. Wary C, Azzabou N, Giraudeau C, et al. Quantitative NMRI and NMRS identify augmented disease progression after loss of ambulation in forearms of boys with Duchenne muscular dystrophy. NMR in biomedicine. 2015;28(9):1150-62.
Figures