3171
Interleaved multi-voxel 1H and 31P MRS for dynamic pH monitoring in the exercising calf muscle at 3 T with dynamic water reference.1NMR Laboratory, Institute of Myology, Paris, France, 2NMR Laboratory, CEA\DRF\IBFJ\MIRCen, Paris, France, 3High Field MR Center, CMPBME, Medical University of Vienna, Vienna, Austria
Synopsis
We extended an interleaved 1H/31P multi-voxel sequence for simultaneous pH monitoring based on carnosine (pH1H, PRESS) and Pi (pH31P, semi-LASER) with a co-localized dynamic water reference (PRESS). Simultaneous pH evaluations were done at 3 T in the soleus and gastrocnemius muscles during a straight-leg plantar flexion paradigm. A pH1H bias of ~0.05 units was removed by using the dynamic water reference. pH1H and pH31P time courses (1-minute temporal resolution) were comparable. Pi and Carnosine (C2-H) resonances broadened during exercise in the gastrocnemius. This method opens the way for new applications in functional studies targeting individual muscles at 3 T.
Introduction
31P MRS allows evaluating energy metabolism and dynamic changes of pH in the muscle during physical effort. 1H MRS can provide cytoplasmic pH information by measuring carnosine, a dipeptide playing a role in pH buffering1. Carnosine-based pH(pH1H) determination is an interesting and complementary method to the 31P MRS evaluation of pH using Pi(pH31P), for instance, in dystrophic myopathies presenting “leaky” membranes2 or during exercise3. Here, we have extended a previous interleaved 1H/31P multi-voxel pulse sequence for simultaneous pH1H and pH31P measurements in muscle4, by adding the acquisition of a dynamic water reference(waterref) co-localized to the water-suppressed(WS) carnosine MRS voxel. We evaluated the impact the that dynamic waterref has on pH1H calculation during an exercise paradigm and compared the pH1H values with their corresponding pH31P pairs.Methods
Experimental Setup.6 men (42±14 y.o.) participated in this study. Experiments were done at 3 T (Siemens Prisma). The RF coil (RAPID Biomedical) combined a 1H birdcage transmitter (20-cm inner diameter), a 1H 18Rx phased-array receiver and a 31P 1Tx/3Rx semi-cylindrical transceiver (11-cm resonator length). The subject’s calf was placed facing the 31P coil and as close as possible to the center of the 1H transmit coil.
Exercise paradigm.
Straight-leg plantar flexions were performed every 3 seconds for 13 min on a pneumatic ergometer, gradually increasing the resistance from ~15% to ~30% of maximum voluntary contraction. MRS lasted 25 min, starting 2 before exercise onset.
Interleaved NMR.
An interleaved multi-nuclear sequence was implemented, performing a WET5 water suppression (80 Hz saturation bandwidth) and acquiring sequentially a WS 1H MR spectrum (PRESS, TE=17.9 ms, 2048 points, 8 kHz bandwidth, carrier frequency at 8 ppm), a 31P MR spectrum (semi-LASER, TE=21.54 ms, 2048 points, 8 kHz bandwidth, centered at the PCr resonance frequency) and a Waterref 1H MR spectrum (PRESS, TE=17.9 ms, 2048 points, 8 kHz bandwidth, carrier frequency at 4.7 ppm) in the same voxel every 3 s (figure 1A). The voxel localization alternated every 3 s between the gastrocnemius (GAS) and the soleus (SOL) muscles (figure 1B).
Data Analysis.
In-house Matlab routines were used for automatic phasing. Individual spectra were then inspected and manual zero and first-order phase correction was performed if needed. Afterwards, spectra were averaged over 1-minute intervals and exported to jMRUI. Spectra were zero-filled (4096 points) and 31P MR spectra were apodized (8 Hz, Gaussian filter) before AMARES quantification. For each voxel, the carnosine and waterref 1H MR spectra were fitted simultaneously. pH1H was calculated from the chemical shift of the carnosine C2-H (δCarn-C2) resonance and corrected for frequency offsets using the water resonance of the respective waterref spectra according to6: $$pH_{1H}=6.81+log_{10}(\frac{8.57-δ_{Carn-C2}}{δ_{Carn-C2}-7.65})$$. For comparison, pH1H was also calculated using the residual water from the WS spectra. The chemical shift difference between Pi and PCr (δPi) was used to calculate pH31P according to7: $$pH_{31P}=6.75+log_{10}(\frac{δ_{Pi}-3.27}{5.69-δ_{Pi}})$$.
Results & Discussion
Figure 2 shows the time series of WS 1H and 31P spectra from both voxels from a single subject. In 3 subjects, a broadening of the carnosine C2-H resonance was observed during exercise in GAS. As shown in figure 3, PCr signal amplitude showed a clear drop of at least 50% in GAS accompanied by a mirroring increase in Pi. In contrast, mild to no changes were observed in SOL, as expected during straight-leg plantar flexions from a study exploring 4 knee angles8. A high variability was observed for carnosine although the average signal amplitude remained relatively stable in both muscles during the paradigm. pH1H values presented a systematic increase of ~0.05 units when using the water residue of the WS spectra instead of waterref (figure 4), demonstrating the interest of a dynamic water reference. The pH1H and pH31P time courses presented similar trends (figure 5, top). In GAS, a pH increase in the first minute of exercise was followed by a steady pH decrease during the following 6 minutes of exercise. pH recovery began in the second or third minute of recovery. In SOL, a pH1H increase was observed only during the second half of the exercise period. Furthermore, when pooling the data of all subjects, a stronger linear correlation between the two pH measuring methods was found (figure 5C-D) in GAS(R²=0.55) than in SOL(R²=0.08). Bland-Altman analysis(figure 5E-F) revealed similar limits of agreement in both muscles but a larger bias in GAS than SOL and the negative mean bias found indicate that in this study pH31P values were lower than their pH1H pairs. Splitting or broadening of the carnosine(C2-H) resonance has been reported after performing a 1-hour street run followed by a toe-hopping exercise3. Here, we found both a widening of the carnosine(C2-H) and Pi resonance in GAS during exercise. Nevertheless, care should be taken when interpreting these results as our spectra were averaged a minute. Future studies could include acquisitions performed in GAS only to double the current temporal resolution.Conclusion
This study showed that adding a dynamic water reference improved pH estimation by 1H MRS and the results were comparable to pH values measured simultaneously on the same voxel with 31P MRS. This method opens the way for new applications of multi-nuclear interleaving in functional studies targeting individual muscles at 3 T.Acknowledgements
No acknowledgement found.References
1. Boldyrev et
al. Physiol Rev. 2013 ; 93: 1803-1845.
2. Reyngoudt et al. NMR Biomed. 2018 ; 31(1).
3. Just Kukurova et al. NMR Biomed. 2016 ; 29: 24-32.
4. Lopez Kolkovsky et al. ESMRMB. 2017, Barcelona; 996.
5. Ogg et
al. J Magn Reson B.1994; 104:1-10.
6. Damon et
al. MRM. 2003; 49:233-240.
7. Moon & Richards. J Biol Chem. 1973; 248: 7276-7278.
8. Niess et
al. NMR Biomed. 2018; 31(6): e3905.
Figures
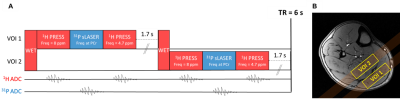
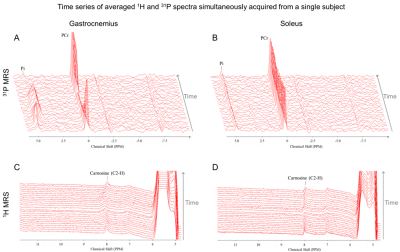
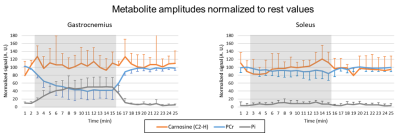
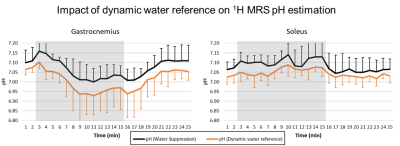
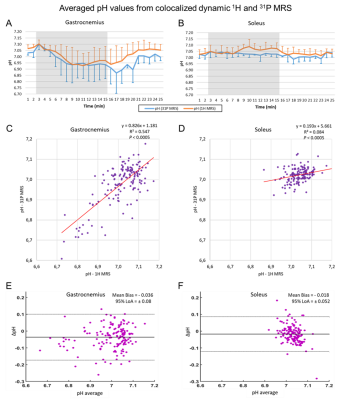
(C,D) Linear correlations and (E,F) Bland-Altman plots between pH1H and pH31P values measured during the exercise paradigm of all subjects. LoA = Limits of agreement.