3167
Altered creatine kinase activity and mitochondrial oxidative capacity in muscular dystrophic mdx mice after repeated muscle contraction
Kihwan Kim1, Yuning Gu1, Yuran Zhu1, Yudu Li2,3, Sherry Huang1, Zhi-Pei Liang 2,3, and Xin Yu1,4
1Biomedical Engineering, Case Western Reserve University, cleveland, OH, United States, 2Electrical and Computer Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 3Beckman Institute for advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 4Case Center for Imaging Research, Case Western Reserve University, cleveland, OH, United States
1Biomedical Engineering, Case Western Reserve University, cleveland, OH, United States, 2Electrical and Computer Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 3Beckman Institute for advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 4Case Center for Imaging Research, Case Western Reserve University, cleveland, OH, United States
Synopsis
This study investigated the creatine kinase activity and the effects of repeated muscle contraction on muscle metabolism in mdx mice, a mouse model for Duchenne Muscular Dystrophy (DMD). Using phosphorous-31 (31P) magnetic resonance fingerprinting and dynamic 31P magnetic resonance spectroscopy, we observed a significant decrease in creatine kinase (CK) reaction rate in mdx mice compared to control mice. Decrease in phosphocreatine (PCr) recovery rate after its depletion by stimulation-induced muscle contraction was also observed in mdx mice. These findings suggest altered mitochondrial function in mdx mice.
Introduction
Duchenne Muscular Dystrophy (DMD), one of the most common muscular dystrophies, is caused by the absence of dystrophin protein which is essential in keeping muscle sarcolemma integrity and stability. The loss of dystrophin leads to muscle degeneration which results in poor muscle function.1–3 Abnormal mitochondrial function is also a prominent characteristics of DMD.1,2,4 Molecular analysis on the skeletal muscle of mdx mouse, a mouse model of DMD, have reported reduced mitochondria DNA derived transcript and proteins, as well as reduced substrate activity in oxidative phosphorylation, suggesting reduced mitochondrial content.5–7 Exercise training can improve muscle strength as well as improve mitochondrial function via mitochondrial biogenesis. In fact, exercise training has shown to increase mRNA expression of mitochondrial markers and transcription factors.8 However, few studies have evaluated the effect of exercise on mdx mice at younger age.Phosphorous-31 magnetic resonance spectroscopy (31P-MRS) provides the opportunity to assess various aspects of muscle metabolism in vivo.9 In particular, 31P-magnetic resonance spectroscopic fingerprinting (31P-MRSF) allows rapid quantification of creatine kinase (CK) reaction rate constant. Dynamic 31P-magnetic resonance spectroscopy (31P-MRS) in combination with a metabolic perturbation protocol (such as exercise), can evaluate mitochondrial oxidative capacity in skeletal muscle through monitoring changes in high-energy phosphate metabolites such as adenosine triphosphate (ATP) and phosphocreatine (PCr).
In this study, evaluated changes in metabolic phenotype in mdx mice in response to muscle contraction induced by electrical stimulation. Our results show significantly decreased CK activity in mdx mice, accompanied by a decrease in mitochondria oxidative capacity and attenuated response to repeated muscle contraction induced by electrical stimulation.
Methods
Experimental protocol: 31P-MRSF and dynamic 31P-MRS studies were performed on C57BL/6 and MDX mice 10 – 12 weeks of age (n = 6 each) using a 9.4T scanner with custom-built 31P saddle coil. All the mice were anesthetized with isoflurane (1.0–1.5%) and placed in a cradle in lateral position. Two needle electrodes were inserted subcutaneously over the third lumbar vertebrae and the greater trochanter, respectively.10 For each mouse, two rounds of electrical stimulation (4 min duration) were performed with a 23-min interval between the two rounds.31P-MRSF: The pseudo-first order forward CK reaction rate (kf,CK) was quantified at baseline and after the two rounds of stimulation using the 31P-MRSF method described previously.9-10 30 fingerprints were acquired for each scan which corresponds to 10 min acquisition. The kf,CK was then determined by matching the averaged fingerprints to a dictionary generated from the Bloch-McConnell simulator.
Dynamic 31P-MRS: The PCr recovery rate and PCr depletion and recovery after electrical stimulation were assessed from dynamic 31P-MRS data acquired using 30° excitation pulses with a TR of 500 ms.10 Each acquisition lasted 25 min covering baseline (5 min), stimulation (4 min) and recovery (16 min) phases. Post processing of the dynamic 31P-MRS data were performed using in-house developed MATLAB-based software.
Results
Figure 1A shows a representative coronal image of mouse skeletal muscle from which the 31P-MRSF and 31P-MRS data were acquired. Figure 1B and 1C show representative 31P-MRSF fingerprint and dynamic 31P spectra. Comparison of kf,CK measured at baseline and after two rounds of stimulation are shown in Figure 2. At baseline, there was no difference in kf,CK between mdx and C57BL/6 (0.41 ± 0.018 vs 0.36 ± 0.034 s-1). Both mice showed significant increase in kf,CK after stimulation-induced muscle contraction. However, mdx mice displayed attenuated kf,CK increase after both rounds of stimulations compared to C57BL/6 (p-value < 0.05).The changes in PCr concentration and recovery rate after each round of stimulation are shown in Figure 3. Overall, there was no difference in PCr recovery rate in mdx mice compared to C57BL/6 mice after the first round of stimulation (0.97 ± 0.20 vs 0.94 ± 0.25 min-1). Initial PCr resynthesis rate was also similar between mdx and C57BL/6 mice (18.72 ± 7.6 vs 19.28 ± 6.01 mM/min). However, after the second round of stimulation, C57BL/6 mice showed a significant increase in PCr recovery rate by ~25% (p < 0.05, Figure 3 A), while mdx mice showed a trend of decrease by ~17%. The initial PCr resynthesis rate decreased by 37% in mdx mice while that of C57BL/6 increased by 17%. Overall, mdx mice displayed significantly decreased PCr recovery rate in round two stimulation compared to C57BL/6 (p-value < 0.05).
C57BL/6 mice showed about 70% PCr depletion after each round of stimulation and recovered to >90% of the baseline level after both rounds. Mdx mice also exhibited similar PCr level depletion and recovery after each round of stimulation (75% PCr depletion and >85% PCr level recovery).
Discussion and conclusion
In this study, we observed similar CK rate constant at baseline and PCr recovery kinetics after first round of stimulation between the mdx mice and the controls. However, mdx mice showed decreased PCr recovery rate while the control mice showed an increase after second round of stimulation. Further, mdx mice also showed attenuated increase in CK activity in response to muscle stimulation. These in vivo observations suggest that while muscle metabolism at baseline remained normal in young mdx mice, the response to repeated muscle stimulation appears abnormal, which could be an early sign of metabolic dysfunction in mdx mice.Acknowledgements
This work was supported by a grant from the National Institute of Health (R01 EB23704).References
- Percival, J. M., Siegel, M. P., Knowels, G. & Marcinek, D. J. Defects in mitochondrial localization and ATP synthesis in the mdx mouse model of duchenne muscular dystrophy are not alleviated by PDE5 inhibition. Hum. Mol. Genet. 22, 153–167 (2013).
- Gaglianone, R. B. et al. Reduced mitochondrial respiration and increased calcium deposits in the EDL muscle, but not in soleus, from 12-week-old dystrophic mdx mice. Sci. Rep. 9, 1–10 (2019).
- Bell, E. L. et al. Mitochondrion PPAR δ modulation rescues mitochondrial fatty acid oxidation defects in the mdx model of muscular dystrophy. 46, 51–58 (2019).
- Vila, M. C. et al. Mitochondria mediate cell membrane repair and contribute to Duchenne muscular dystrophy. 330–342 (2017) doi:10.1038/cdd.2016.127.
- Hughes, M. C. et al. Early myopathy in Duchenne muscular dystrophy is associated with elevated mitochondrial H2O2 emission during impaired oxidative phosphorylation. J. Cachexia. Sarcopenia Muscle 10, 643–661 (2019).
- Turk, R. et al. Muscle regeneration in dystrophin-deficient mdx mice studied by gene expression profiling. BMC Genomics 6, 1–15 (2005).
- Moore, T. M. et al. Mitochondrial Dysfunction Is an Early Consequence of Partial or Complete Dystrophin Loss in mdx Mice. Front. Physiol. 11, 1–15 (2020).
- Moll, K. et al. Comparison of metabolic adaptations between endurance- and sprint-trained athletes after an exhaustive exercise in two different calf muscles using a multi-slice 31 P-MR spectroscopic sequence. NMR Biomed. 31, 1–15 (2018).
- Kim, K. et al. Quantification of creatine kinase reaction rate in mouse hindlimb using phosphorus-31 magnetic resonance spectroscopic fingerprinting. NMR Biomed. 1–11 (2020) doi:10.1002/nbm.4435.
- K. Kim, Y. Gu, G. Kim, M. Wong, B. Clifford, S. Huang, Z. Liang, and X. Yu “Repeated muscle contraction increases creatine kinase reaction rate and shortens phosphocreatine recovery in mouse skeletal muscle,” ISMRM 2020
Figures
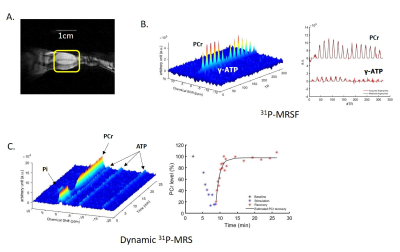
Figure 1. (A) Coronal image of mouse
hindlimb with a 1cm long landmark to display the length and location of 31P-
saddle coil; (B) 3D 31P-MR
fingerprints and 2D 31P-MR fingerprints matched to a dictionary
entry (C) 3D and 2D dynamic 31P-MR
spectra
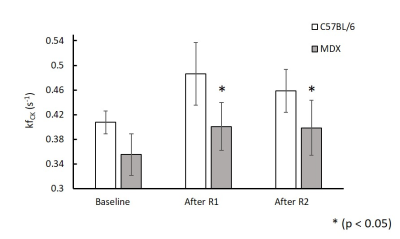
Figure 2. In vivo
mean CK reaction rate at baseline, after round 1 and round 2 stimulations in C57BL/6 and mdx mice
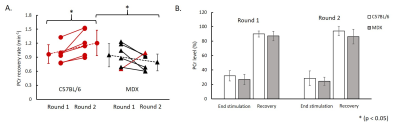
Figure 3. (A) PCr
recovery rate between round 1 and round 2 stimulations (red color indicates increase in PCr recovery
rate and black color indicates decrease in PCr recovery rate); (B) PCr level at
the end of stimulation-induced muscle contraction and at after 16 min recovery.