3164
The Changes in Longitudinal ALFF and ReHo Values of Methamphetamine Abstinence Subjects Based on Harvard-Oxford Atlas1Department of Radiology, the Second Xiangya Hospital of Central South University, Changsha, China, 2MR Scientific Marking, Siemens Healthcare Ltd., Wuhan, China
Synopsis
This study aimed to explore the changes of amplitude low-frequency fluctuation (ALFF) values nearly one-year before and after abstinence in patients with Methamphetamine Abstinence (MA), and to investigate potential imaging markers related to withdrawal based on Harvard-Oxford Atlas. Compared with short-term abstinence group, right middle frontal gyrus (8) and right inferior frontal gyrus, pars triangularis (10) had significantly higher ALFF value at long-term abstinence group. The ALFF value of the two regions may be a new biomarker which can reflect the impact of withdrawal on brain function.
Introduction
Methamphetamine (MA), that becomes one of the most rapidly growing illicit drug, is a highly addictive psychostimulant drug that can affect the central nervous system (CNS) 1, 2. Researches have shown that MA abuse caused comprehensive changes to brain structures and functions, while the brain function can be improved to a certain extent after a period of withdrawal3. In addition, the neuronal function changes can be reflected with blood oxygenation level-dependent (BOLD) signals by analyzing the change of hemodynamic, and the amplitude low-frequency fluctuation (ALFF) and regional homogeneity (ReHo) are two indicators that can represent the spontaneous neuronal activity of the regional brain area4. Therefore, the purpose of this study was to explore the changes of ALFF and ReHo values nearly one-year before and after abstinence and to investigate potential imaging markers related to withdrawal.Methods and materials
The study was approved by the ethics committee of the Second Xiangya hospital of Central South University (Hunan, China). All participants provided written informed consent prior to entering into the study. Our study included 63 MA-dependent participants and they were collected from drug rehabilitation centers in Changsha, Zhuzhou and Yueyang (Hunan, China). During abstinence, the participants were treated without MA, but with medicine, education, and physical exercise. 13 participants were excluded due to poor MR image quality caused by severe head motion. Hence, the study included 50 MA-abstinent right-handed people and collected the demographic characteristics of short-term and long-term withdrawal of each person. According to withdrawal time, they were divided into short-term abstinence group (< 1 year) and long-term abstinence group (> 1 year). All imaging data were acquired on a 3T MRI scanner (MAGNETOM Skyra, Siemens Healthcare, Erlangen, Germany) with a 32-channel head coil. The MRI scanning included T1w three-dimensional magnetically prepared rapid acquisition gradient echo (3D MPRAGE) sequence (176 sagittal slices, slice thickness=1 mm, gap=0 mm, field of view (FOV)=256 mm×256 mm, repetition time (TR)=1450 ms, echo time (TE)=2.03 ms, inversion time (TI)=900 ms, flip angle=30°, and voxel size=1×1×1mm3) and resting-state fMRI sessions (36 axial slices, thickness=4 mm, FOV=220 mm×220 mm, TR=2000 ms, TE=30 ms, flip angle=80°, and 225 volumes). Data Processing Assistant for Resting-State fMRI (DPABI, 4.3, Advanced edition) software (http://rfmri.org/dpabi) based on Matlab 2016b was used to conduct the MR imaging preprocessing. Furthermore, the whole-brain ALFF value of short-term and long-term abstinence participants were extracted separately from the Harvard Oxford atlas (HOA). The ALFF and ReHo values between the short-term and long-term abstinence group were evaluated using the paired sample t test, and the p value was corrected for multiple comparisons by FDR correction (P<0.05).Results
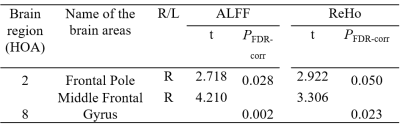
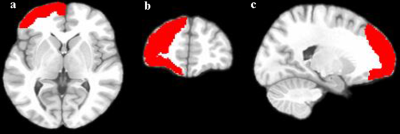
50 subjects (34 male and 16 female) with short-term abstinence (25.22±14.99 days) first as well as long-term abstinence (329.33±90.18 days) later were included. The detailed information are summarized in Table 1. Brain regions’ changes based on HOA in ALFF and ReHo values (FDR-corrected P<0.05) between short-term and long-term abstinence group are shown in Tables 2 and Figure 1. In comparison with the short-term abstinence group, the long-term abstinence group had significantly higher ALFF and ReHo values in the following brain regions: right frontal pole (2) and right middle frontal gyrus (8).Discussion
This study compared ALFF and ReHo values of short-term and long-term abstinence groups to investigate the characteristics of ALFF and ReHo in abstinence subjects and to examine ALFF and ReHo as possible biomarkers for MA long-term withdrawal. As the results shown, there were significant differences in right frontal pole (2) and right middle frontal gyrus (8) between the two groups. Early study indicated that the middle frontal gyrus is an important brain area that inhibits impulsivity, and the long-term MA use will damage the function of this area and reduce self-control ability, resulting in the inability to suppress the craving for drugs 5, 6. Therefore, it is possible that the abstinence caused the changes of ALFF value of middle frontal gyrus.Conclusion
In summary, this study found right middle frontal gyrus changes in MA-dependent subjects with long-term abstinence in comparison with short-term abstinence group. Hence, this study not only provides an imaging basis for revealing the neural mechanism of long-term abstinence of MA, but also provides the potential imaging markers related to withdrawal.Acknowledgements
No acknowledgement found.References
1. Methamphetamine- and trauma-induced brain injuries: Comparative cellular and molecular neurobiological substrates. Biological psychiatry. 2009;66:118-127
2. Panenka WJ, Procyshyn RM, Lecomte T, MacEwan GW, Flynn SW, Honer WG, et al. Methamphetamine use: A comprehensive review of molecular, preclinical and clinical findings. Drug and alcohol dependence. 2013;129:167-179
3. Choi JK, Lim G, Chen YI, Jenkins BG. Abstinence to chronic methamphetamine switches connectivity between striatal, hippocampal and sensorimotor regions and increases cerebral blood volume response. NeuroImage. 2018;174:364-379
4. Tian X, Wei D, Du X, Wang K, Yang J, Liu W, et al. Assessment of trait anxiety and prediction of changes in state anxiety using functional brain imaging: A test-retest study. NeuroImage. 2016;133:408-416
5. Salo R, Ursu S, Buonocore MH, Leamon MH, Carter C. Impaired prefrontal cortical function and disrupted adaptive cognitive control in methamphetamine abusers: A functional magnetic resonance imaging study. Biological psychiatry. 2009;65:706-709
6. Sabrini S, Wang GY, Lin JC, Ian JK, Curley LE. Methamphetamine use and cognitive function: A systematic review of neuroimaging research. Drug and alcohol dependence. 2019;194:75-87