3161
Clavulanic Acid Alters Functional Connectivity of the Anterior Cingulate Cortex in Subjects with Cocaine Use Disorder: A Pilot fMRI Study1Psychiatry, Lewis Katz School of Medicine at Temple University, Philadelphia, PA, United States, 2Center for Substance Abuse Research, Lewis Katz School of Medicine at Temple University, Philadelphia, PA, United States, 3Psychiatry, Beth Israel Deaconess Medical Center, Boston, MA, United States, 4Psychiatry, Harvard Medical School, Boston, MA, United States
Synopsis
Unlike alcohol and nicotine, there is no FDA-approved pharmacological treatment for cocaine use disorder (CoCUD). The purpose of this study was to investigate clavulanic acid (CLAV), a GLT-1 activator, for its potential to treat CoCUD. Resting state fMRI was used to assess changes in the anterior cingulate cortex (ACC) functional connectivity with repeated CLAV for 10 days. CLAV altered the connectivity of ACC with default mode network, motor control, and addiction cue reactivity related regions. This pilot study supports the development of CLAV for CoCUD treatment.
Background
There is an urgent need for a pharmacological treatment for Cocaine Use Disorder (CoCUD). To date, there is no FDA-approved medication to treat CoCUD. Clavulanic acid (CLAV) has potential for treating CoCUD via a novel compelling mechanism of action. CLAV is a beta-lactam activator of the glutamate transporter GLT-1 (excitatory amino acid transporter, EAAT2), which could modify neural excitatory activity. In the present proof of concept pilot study, we used resting state functional Magnetic Resonance Imaging (rsfMRI) to test the hypothesis that CLAV will modify the functional connectivity in craving-associated networks.Methods
Four subjects with CoCUD in early remission participated in this study. During 2 separate MRI scanning visits, we used rsfMRI to evaluate changes in functional connectivity between baseline (before CLAV) and after 10 days of repeated CLAV administration 500 mg/day for 10 days. Subjects were instructed to relax, keep eyes open and stare at a cross during 2 rsfMRI runs of 6 minutes each. MRI scans were conducted using a Siemens MAGNETOM Prisma 3-Tesla whole-body MRI scanner. A localizer scan was performed to define placement for the subsequent scans. A T1-weighted 3D MPRAGE scan was performed to facilitate registration of the rsfMRI data into the MNI space. A BOLD-EPI with multiband factor of 3. 60 axial slices with gap of 0.1mm, 2.0 mm thickness sequence was used for rsfMRI (TR/TE= 2000/29 ms, 2 x 2 x 2 mm3 voxel resolution, 180 volumes per run). rsfMRI data preprocessing and analysis were performed using the Statistical nonParametric Mapping (SnPM) (http://warwick.ac.uk/snpm) software package. We performed seed-based functional connectivity analysis using the anterior cingulate cortex (ACC), defined using the Harvard-Oxford Cortical Structural atlas, as the seed. Nonparametric tests comparing baseline to 10-day CLAV administration rsfMRI used 5000 permutations, FDR of 0.01, and cluster level of 100.Results
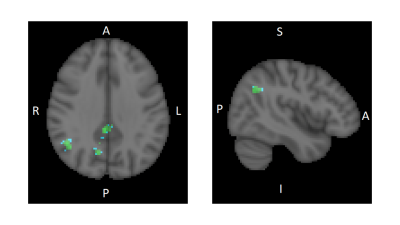
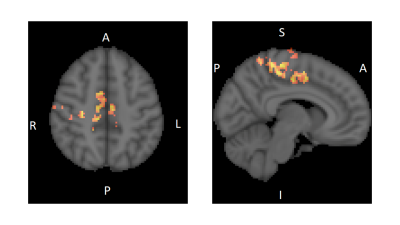
CLAV, given for 10 days, decreased the connectivity between the ACC and the Angular gyrus, Superior Lateral Occipital gyrus, Precuneus, and Posterior Cingulate Cortex regions, the latter three being nodes of the default mode network. (Fig. 1) CLAV increased the connectivity between the ACC and the Paracentral lobe, Supplementary Motor Area (SMA), and dorsal anterior cingulate regions. (Fig. 2)Discussion
This proof of concept pilot study suggests that CLAV modulates resting state functional connectivity of the ACC with several brain regions involved in modulating behavior in cocaine addiction. The ACC is implicated in sensory-motor integration, cognitive control and reward functions. Results suggest that repeated CLAV administration may decrease connectivity of ACC with a parietal associative region (angular gyrus) and default mode network regions. In behavioral addiction studies, the angular gyrus is linked to cue reactivity. A meta-analysis showed cue reactivity is associated with increase neural activation in the angular gyrus and precuneus in behavioral addictions (1). In our study, CLAV reduced resting state functional connectivity in these regions, suggesting that CLAV may reduce addiction cue reactivity. CLAV increased connectivity with sensory-motor control regions and SMA. SMA is known to be involved in voluntary internally generated motor function and movement preparation. Thus, CLAV related increases in functional connectivity suggest increased behavioral motor control.Conclusion
This pilot study identified changes in functional connectivity of brain networks involved in addiction behaviors after repeated CLAV administration in CoCUD. These data expands the body of knowledge on the effects of beta-lactam and glutamate transporter drugs on brain function in addiction. Furthermore, they are indicative of a potential efficacious pharmacological treatment for CoCUD to prevent relapse.Acknowledgements
This work was supported by NIH grants U54 DA039002 and T32 DA007237.
References
1. Starcke K, Antons S, Trotzke P, Brand M. Cue-reactivity in behavioral addictions: A meta-analysis and methodological considerations. J Behav Addict. 1; 7(2):227-238 (2018).
Figures