3155
Global Connectivity of the Cerebellum Predicts Slow Wave Sleep Improvement: A Randomized Controlled Acupuncture Trial1Department of Radiology, Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China, 2Department of Neurosurgery, Pennsylvania State University College of Medicine, Hershey, PA, United States, 3Department of Acupuncture, Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China, 4Department of Otolaryngology, Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China, 5Department of Radiology, Pennsylvania State University College of Medicine, PA, USA, Hershey, PA, United States, 6Department of Neurology, Dongfang Hospital, Beijing University of Chinese Medicine, Beijing, China, 7Department of Radiology, Pennsylvania State University College of Medicine, Hershey, PA, United States, 8Department of Radiology, Xuanwu Hospital, Capital Medical University, Beijing, China
Synopsis
The cerebellum is an important brain structure for sleep. We identified a global connectivity mechanism with which the cerebellum coordinates and controls sleep-related networks. Global connectivity of the cerebellum showed a highly selective relationship with individual differences in slow-wave sleep (SWS) improvements, after both verum and sham acupuncture treatments. Cerebellar connectivity with the thalamus differed significantly between treatment types. Highlighting its ability to distinguish amongst processes central to sham and verum treatments. Our findings suggest a particular architecture for the cerebellum: a flexible global hub with a brain-wide influence, supporting both circadian rhythms and sleep homeostasis.
INTRODUCTION
The cerebellum is an important brain structure for sleep1. There’s a dearth of data covering its numerous contributions (i.e., its activity and connectivity patterns) to various sleep stages. Here, we focused on the global brain connectivity of the cerebellum in patients with chronic insomnia disorder (CID).1 We investigated their cerebellar connectivity using acupuncture treatment, and weighted degree centrality (DC), a well established resting state functional fMRI metric. Acupuncture treatment is known to relieve hyperarousal, which improves sleep quality in CID patients2. This study tested the hypothesis that the efficacy of accuputure treatments can be measured using global connectivity chages in specific cerebellar regions.METHODS
Study participants: Twenty-six CID patients (20 females, mean age=58.31 ± 5.56 years) participated in the study (Table 1). Patients met the criteria of Polysomnography (PSG). All CID patients were right-handed and had no history of other general (e.g., cardiovascular, endocrine, etc.) or neurologic medical problems. Except for mild to moderate apnea, hypopnea (AHI, <15), and periodic limb movements during sleep (PLMS, total PLM index < 25/h). None of the patients had any other sleep problems. Acupuncture treatment: CID patients were randomly divided into verum acupuncture and sham acupuncture groups. Acupoints related to insomnia were chosen uniformly. In the sham group, acupoints were 0.5cm to the right of verum acupoints. Data processing: Resting-state fMRI data preprocessing, degree centrality, and functional connectivity computations were performed using the Dpabi software.RESULTS
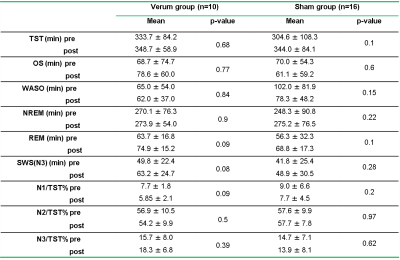
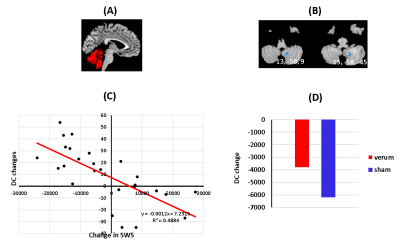
There were significant improvements in sleep and neuropsychological assessment scores, for both verum and sham groups, after treatment. Behavioral improvements were not significantly different between the two groups (see Table 1 in Figure 1). We found decreased degree centrality within the right cerebellum (8, 9), which was negatively correlated with improved SWS measurements (voxel ≥ 5, p < 0.00, FDR corrected). Again, the cerebellar DC changes were not significantly different between the two groups (Figure 2). We next sought to more thoroughly examine the source of this DC effect— to determine whether it was truly global in nature or if it reflected a dominant effect amongst a subset of cerebellar connections, and whether the right cerebellar DC effect was driven by its connectivity to the hypothalamus. We discovered that the functional connectivity (FC) changes, between the right cerebellum (the seed) and the right thalamus, were predictive of sleep improvements in the combined group. Additionally, these FC values were significantly differed between the both groups (Figure 3).DISCUSSIONS
Advances in brain connectivity analytical methods have made it possible to identify hubs containing the brain's most globally connected regions. Our results suggest that acupuncture treatments may alter the global connectivity of the cerebellum—improving sleep metrics in both groups. There are extensive connections between the cerebellum, and thalamus, 3, 4 a critical structure involved in sleep-wake modulation.5 We also found that improved sleep quality, due to acupuncture treatment, induces specific connectivity changes within the cerebellar-thalamic network. These findings re-conceptualize cerebellum’s role as a functional hub, capable of controlling circadian rhythms and sleep homeostasis across the entire brain.CONCLUSIONS
Our analysis revealed that the DC of most regions in the cerebellum could not reliably predict sleep improvement, as the DC of only a single region— right cerebellum (8, 9) —could do so. This points to the possibility that there are specific regions within the cerebellum that use brain wide connectivity as a central mechanism for regulating sleep behavior.1 The connectivity between the cerebellum and thalamus is modulated by acupuncture treatment and (to a lesser extent) placebo manipulation. Delineating cerebellar connectivity is pertinent, not only for understanding the circadian network in humans, but for developing objective markers for CID and other sleeping disorders.Acknowledgements
We thank all authors of the included studies. We especially thank Center for NMR Research of Penn State University College of Medicine for all staffs' kind help and suggestion.References
1. Canto, C. B., Onuki, Y., Bruinsma, B., et al. The Sleeping Cerebellum. Trends in neurosciences, 2017;40(5), 309-323.
2. Asanuma, C., Thach, W. T., Jones, E. G. Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. Brain Res, 1983;286(3), 237-265.
3. Hendry, S. H., Jones, E. G., Graham, J. Thalamic relay nuclei for cerebellar and certain related fiber systems in the cat. J Comp Neurol, 1979;185(4), 679-713.
4. Gent, T. C., Bassetti, C., Adamantidis, A. R. Sleep-wake control and the thalamus. Curr Opin Neurobiol, 2018;52, 188-197.
Figures