3128
Temporal filtering affects time-varying functional connectivity metrics of the human brain
Francesca Saviola1, Stefano Tambalo1, and Jorge Jovicich1
1CIMeC, Center for Mind/Brain Sciences, University of Trento, Rovereto (TN), Italy
1CIMeC, Center for Mind/Brain Sciences, University of Trento, Rovereto (TN), Italy
Synopsis
The use of temporal filtering is still controversial in resting-state brain functional MRI. In this study, we show that time-varying connectivity patterns largely depend on the type of temporal filtering applied to the data. Our results highlight the importance of properly considering and reporting the pre-processing pipeline details as they may significantly impact on obtaining reproducible findings, especially in clinical studies.
Background
The application of temporal filtering in the pre-processing of fMRI data is aimed at improving neural specificity and temporal signal-to-noise ratio by disentangling unstructured/physiological noise from neural signals in the time-domain. However, if for task-based fMRI fine-tuning of the filter is determined by the stimulation paradigm, in the case of resting-state fMRI (rs-fMRI) there are no a-priori cutoff values to be chosen by design, therefore it’s not trivial to define the optimal bandwidth attenuating noise while preserving meaningful information from the signal. Hence, the issue of what can be defined as “noise” strikes back: historically, noise is recognized in both low frequencies, attributable to hardware-related issues (e.g. scanner drifts), and higher frequencies, associated with physiological fluctuations (e.g. heartbeat, respiration). However, the use of high-frequency attenuation is still controversial[1]. Undoubtedly, accurate filtering of higher frequencies content enables potential mitigation of short-lived artifacts such as motion- and cardiac-related[2] fluctuations, but there is evidence that a sub-optimal usage of a low-pass filter reduces reliability in functional connectivity due to a weak attenuation of noisy signals[3,4]. This poses an issue about the effects of temporal filtering, especially in the case of time-varying connectivity (TVC) analysis, where we try to disentangle the dynamic properties of physiological fluctuations from neural recurrent states. In this study, we compare how different temporal filtering approaches applied to a standard rs-fMRI protocol of TR 2 s affect TVC metrics in order to assess the reproducibility of TVC findings.Materials and Methods
A total of 17 Healthy Controls (9 males; age (mean, SD) 24±4 years) underwent 3T MRI (MAGNETOM Prisma, Siemens Healthcare, Erlangen, Germany) using a 64-channel head/neck receiver RF coil. Structural T1-weighted MPRAGE (TR=2.31 s, TEs=3.48 ms, voxel-size=1 mm3 iso) and rs-fMRI (TR=2 s, TE=28 ms, voxel-size=3 mm3 iso) images were used to test the effects of different preprocessing pipelines on TVC estimations. MRI data were pre-processed using two different workflows (Figure 1). Common pre-processing steps included: (1) Slice timing and head motion correction; (2) co-registration of T1-weighted image to rs-fMRI time series; (3) T1-weighted image tissues segmentation; (4) temporal regression of 6 head motion parameters, white matter, and cerebrospinal fluid signals; (5) normalization to standard MNI template space; (6) spatial smoothing with 2 voxels full width half maximum Gaussian kernel size. Together with standard pre-processing steps, the two pipelines differed in: (i) no temporal filtering (prep1); (ii) band-pass temporal filtering [0.01-0.1 Hz] (prep2). For TVC analysis, innovation-driven co-activation patterns (iCAPs) were estimated in Matlab (https://c4science.ch/source/iCAPs/)[5]. Steps included: (i) deconvolution of the hemodynamic response applied through spatio-temporal regularization to then derive total activation signal; (ii) changes in total activation from each subject (recognized as transients) were concatenated and tested with temporal K-means clustering to derive co-occurrent transitions in neural BOLD signals: the iCAPs. The optimum number of clusters, determined by consensus clustering, for both pipelines was set to 16; (iii) As a last step, timecourses of all iCAPs were extracted by means of transient-informed regression, and TVC metrics were derived. After iCAPs estimation, in order to separate noisy iCAPs from underlying resting-state networks, iCAPs were tested for their correlation (threshold of r-value > 0.3) to labeled networks[6]; two iCAPs out of 16 were identified as of no interest (r-value < 0.3) and discarded. Duration and coupling measures between pipelines were compared using a Wilcoxon signed-rank test and corrected for multiple comparisons.Results
Duration of iCAPs, detected as percentage of the total non-motion scanning time spent in the network for each subject, significantly differed between prep1 and prep2. Cingulum (p-valueFDR=3.1e-05), auditory/sensorimotor (p-valueFDR=0.02), posterior (p-valueFDR=0.01) and whole default mode network (p-valueFDR=1.5e-04), subcortical (p-valueFDR=0.02) and amygdala/hippocampal (p-valueFDR=0.02) networks show an increase in total temporal duration percentage in prep1 compared to prep2. Instead, primary visual (p-valueFDR=3.3e-07), dorsal anterior cingulate/dorsal prefrontal cortex (p-valueFDR=8.8e-04) and anterior Insula (p-valueFDR=3.7e-06) show a decrease in total temporal duration percentage in prep1 compared to prep2. Temporal coupling of networks significantly differed between prep1 and prep2 (p-valueFDR<0.05), highlighting differences in brain networks interactions. Several networks show coupling differences, in prep1 duration is longer mainly for positive coupling (9 couplings, 7 anti-couplings), whereas the pattern is reversed for shorter duration (5 couplings, 11 anti-couplings).Discussion and Conclusions
Previous studies compared clinical populations and healthy controls by means of TVC metrics aiming at detecting dynamic functional signatures of pathologies[7,8]. Given the novelty of the TVC technique, we aimed at assessing the effects of temporal filtering on dynamic metrics. Our study shows that a standard band-pass filter, based on the spectral features of the hemodynamic response function[9], can largely affect TVC metrics, such as the duration of different core networks and their relative coupling. In other words, depending on the temporal filter choice, the same raw data can lead to different dynamic connectivity features that could be related to behavior, cognition, or disease. To support reproducible research, our findings stress the importance of considering and properly reporting pre-processing pipeline details in TVC studies.Acknowledgements
This study was partially funded by funding provided by the Dipartimento di Eccellenza project, 232 law of 2016.References
- Boubela, R. N., Kalcher, K., Huf, W., Kronnerwetter, C., Filzmoser, P., & Moser, E. (2013). Beyond noise: using temporal ICA to extract meaningful information from high-frequency fMRI signal fluctuations during rest. Frontiers in human neuroscience, 7, 168.
- Constable, R. T., & Spencer, D. D. (2001). Repetition time in echo planar functional MRI. Magnetic Resonance in Medicine: An Official Journal of the International Society for Magnetic Resonance in Medicine, 46(4), 748-755.
- Shirer, W. R., Jiang, H., Price, C. M., Ng, B., & Greicius, M. D. (2015). Optimization of rs-fMRI pre-processing for enhanced signal-noise separation, test-retest reliability, and group discrimination. Neuroimage, 117, 67-79.
- Noble, S., Scheinost, D., & Constable, R. T. (2019). A decade of test-retest reliability of functional connectivity: A systematic review and meta-analysis. Neuroimage, 203, 116157.
- Karahanoğlu, F. I., & Van De Ville, D. (2015). Transient brain activity disentangles fMRI resting-state dynamics in terms of spatially and temporally overlapping networks. Nature communications, 6(1), 1-10.
- Shirer, W. R., Ryali, S., Rykhlevskaia, E., Menon, V., & Greicius, M. D. (2012). Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cerebral cortex, 22(1), 158-165.
- Zöller, D., Sandini, C., Karahanoğlu, F. I., Padula, M. C., Schaer, M., Eliez, S., & Van De Ville, D. (2019). Large-scale brain network dynamics provide a measure of psychosis and anxiety in 22q11. 2 deletion syndrome. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 4(10), 881-892.
- Bommarito, G., Tarun, A., Farouj, Y., Preti, M. G., Petracca, M., Droby, A., ... & Van De Ville, D. (2020). Functional network dynamics in progressive multiple sclerosis. medRxiv.
- Josephs, O., & Henson, R. N. (1999). Event-related functional magnetic resonance imaging: modelling, inference and optimization. Philosophical transactions of the royal society of london. series b: biological sciences, 354(1387), 1215-1228.
Figures
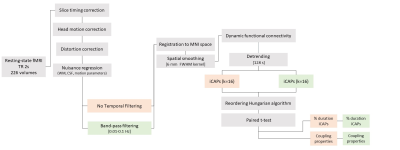
Resting-state fMRI pre-processing pipeline description with two temporal filtering options, without and with band-pass filtering. Steps that are common to both workflows are depicted in grey, prep1 (no temporal filtering) in red and prep2 (band-pass filtering) in green.
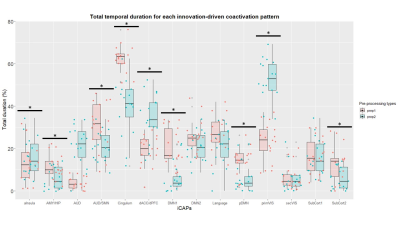
iCAPs total duration percentage from two pre-processing pipelines (same data). PrimVIS: primary visual, SecVIS: secondary visual, aInsula: anterior insula, Language: language network, dACC/dPFC: dorsal anterior cingulate cortex/dorsolateral prefrontal cortex, pDMN: posterior default mode network, DMN: whole default mode network AUD/SM: auditory/sensorimotor, AUD: auditory, AMY/HIP: amygdala/hippocampus, SubCort: subcortical network. Asterisk denotes significant differences (p-valueFDR< 0.05)
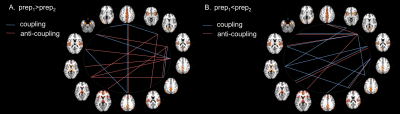
iCAPs coupling differences when the same data undergoes different temporal filtering. Red and blue denote co-activations with the same or opposite signs, respectively. A) Couplings with significantly longer duration in prep1. B) Couplings with a significantly shorter duration in prep1. Couplings were measured in terms of the percentage of total scanning time of the two co-occurrent iCAPs.