3122
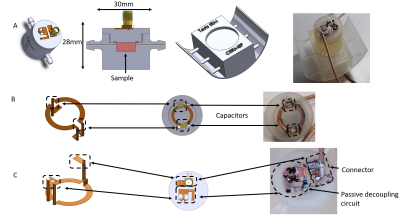
Compact MRI bioreactor for real-time monitoring 3D printed tissue-engineered constructs.1Université Claude Bernard Lyon 1, VILLEURBANNE, France, 2INSA LYON, VILLEURBANNE, France, 3Ecole Centrale Lyon, Ecully, France, 4CNRS, VILLEURBANNE, France, 5AMPERE UMR 5005, VILLEURBANNE, France, 6Université de Lyon, VILLEURBANNE, France, 73d.FAB, VILLEURBANNE, France, 8CPE Lyon, VILLEURBANNE, France, 9ICBMS, VILLEURBANNE, France, 10UMR 5246, VILLEURBANNE, France
Synopsis
Tissue engineering for regenerative medecine is a growing field which faces structural and functional challenges at different scales. Real-time quantitative 3D characterization of tissues both in vitro and in vivo would help biologists assessing their methods. Magnetic Resonance Imaging (MRI) offers the possibility to perform such characterization non-invasively. We propose here a 7T MRI coil integrated within a perfusion tissue engineering bioreactor to perform tissue assessments throughout its growth. The MRI bioreactor is built using 3D printing and plastronics. This works resulted in a successful observation of a bioprinted tissue with a 75 µm in plane resolution.
Introduction
Tissue engineering and regenerative medecine have been developing for a few decades now and the number of applications is growing 1 From cartilage to skin, a wide range of tissues are currently being studied and constructed. However, along with the increasing number of applications, comes an increasing number of different parameters of interest for tissue characterization depending on its application. With multiscale and multiparametric acquisitions, MRI is best suited for characterizing various types of key properties in tissue development. Recent advances 2 highlighted the interest of real-time monitoring of tissues during their growth. As a non-invasive and non-ionizing modality, MRI also qualifies as a potential candidate for this task. In this context, the development of dedicated MRI apparatus specifically designed for this field becomes mandatory. Here we study the feasibility of integrating an MRI sensor directly into the bioreactor to monitor in real time cell fate and tissue growth in 3D printed tissue-engineered constructs.Method
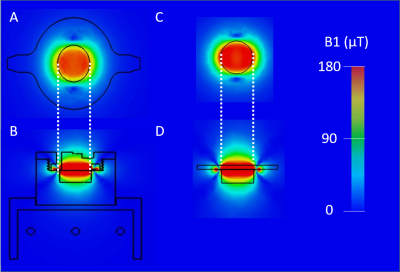
Different MRI setups have been designed for small tissue samples over the years 3,4. The present case is different as the tissue is living and growing while being probed, to our knowledge, only one group 5 tackled this issue. This implies the combined integration of a coil and a non-magnetic bioreactor within the bore of the scanner. Merging these two functions can be done by addition with a conventional FR4-based circular coil placed on top of an operating bioreactor. This method is simple to setup, on the downside, the loss in coil sensitivity due to the bioreactor being placed between the sample and the coil can be detrimental. In addition, this loss would be bioreactor-dependent, leading to poor setup transferability between applications. On the other side, another solution exists with the integration of the coil within the bioreactor itself. This integration has been made possible by the previous progresses in plastronics techniques 6. Once the design of the bioreactor was conceived in SOLIDWORKS, simulations were conducted using CST MICROWAVE STUDIO. The purpose was to evaluate the influence of the dielectric environment of the coil on its sensitivity and field homogeneity compared to a conventional FR4 coil that would be considered as a reference (figure 2).A 12mm reception circular coil was designed and integrated within a perfusion bioreactor based on a previous design 7. This type of bioreactor is commonly used in tissue engineering 8 and fits the scope of this work as it contains no active parts. The cell culture medium is flowing through to the sample via 2 inlets connected to external fluidic circulation pumps. The bioreactor was 3D printed using high temperature resin. The copper coil was then deposited on the surface of the bioreactor following the method described in 6. X-ray fluorescence measurements showed a copper thickness of 45µm and a 4-points method placed the resistivity around 1.75×10-8 Ω.m. As illustrated in figure 1, the coil and its passive decoupling circuit were conceived in 3D using via through the wall of the bioreactor. The 300 MHz tuning/matching of the coil was done remotely with a tuning box with two trimmer capacitors, one in parallel (tuning) and one in series (matching). Finally, a mechanical support was built to allow the bioreactor to fit inside a 36mm radius birdcage transmission coil.
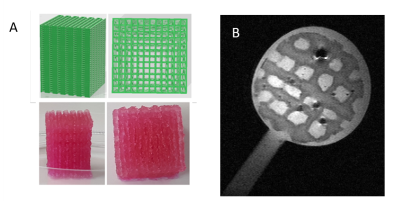
Bench characterization of the coil included unloaded/loaded quality factor measurements. For comparison purposes, bench characterization has also been conducted on a coil made with copper tapped on an FR4 plate. Additional characterization of the bioreactor coil has been conducted in imaging conditions with a 7T Bruker MRI scanner. SNR measurements were performed on an echographic gel using 3D Flash sequence with following parameters: matrix 128×128×64, a FOV 19.2×19.2×9.6 mm3 i.e.150µm3 isotropic resolution, TR 33.81ms, TE 15ms, 16 averages for a total acquisition time of 74min. As proof of concept for our bioreactor, a 2D turborare MRI acquisition was performed on a tissue engineering construct with following parameters: matrix 256×256×64, a FOV 19.2×19.2mm2 i.e. 75µm2 in-plane resolution, 12 slices of 500 µm thickness, TR 3000ms, TE 15ms, Rare factor 8, 4averages for a total acquisition time of 4min 48s
Results
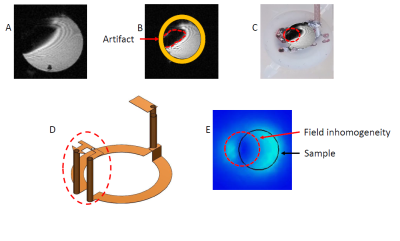
For long TE sequences, an important image artifact appeared. Numerical simulations showed that this is due to the 3D structure of the bioreactor coil leading to a parasitic loop causing field extinction near its location (figure 4). This parasitic loop could explain that the SNR was 1.36 times lower than the SNR obtained with a larger commercial single loop coil available at the imaging facility (16 mm diameter). An in-plane 75 µm² resolution on the bioprinted sample was achieved with the bioreactor.Conclusion
The 3D integration of the MRI coil to the bioreactor needs to be improved to open the way for the first real-time monitoring of a constructing tissue. A validation step for this integration would require a B1 mapping using recent methods 9. The integration of a piezoelectric vibrating membrane is also studied for elastography purposes. In conclusion, this work demonstrates the feasibility of an integrated coil inside a bioreactor and paves the way for a democratization of the MRI tool for biologists using tissue-tailored fully integrated MRI- bioreactors.Acknowledgements
This work was supported by a grant from the Agence National de la Recherche (Estimate Project N° ANR-18-CE19-0009-01). The financial support provided by Ingénierie@Lyon, member of the Carnot Institutes Network (Metafab 3D project) for the postdoctoral scholarship of Dr T. Gerges is also acknowledged. Moreover, the role of CERMEP - Imagerie du vivant and Radu Bolbos and is acknowledged.References
1. Shafiee, A. & Atala, A. Tissue Engineering: Toward a New Era of Medicine. Annu. Rev. Med. 68, 29–40 (2017).
2. Welter, J. F. & Baskaran, H. Monitoring and real-time control of tissue engineering systems. in Principles of Tissue Engineering 1459–1467 (Elsevier, 2020). doi:10.1016/B978-0-12-818422-6.00079-4.
3. Meadowcroft, M. D. et al. Direct magnetic resonance imaging of histological tissue samples at 3.0T. Magn. Reson. Med. 57, 835–841 (2007).
4. Sengupta, S. et al. High resolution anatomical and quantitative MRI of the entire human occipital lobe ex vivo at 9.4 T. NeuroImage 168, 162–171 (2018).
5. Othman, S. F., Wartella, K., Khalilzad Sharghi, V. & Xu, H. The e -Incubator: A Magnetic Resonance Imaging-Compatible Mini Incubator. Tissue Engineering Part C: Methods 21, 347–355 (2015).
6. Gerges, T. et al. 3D Plastronics for Smartly Integrated Magnetic Resonance Imaging Coils. Front. Phys. 8, 240 (2020).
7. Pourchet, L. LARGE 3D BIOPRINTED TISSUE_ HETEROGENEOUS PERFUSION AND VASCULARIZATION. 13.
8. Gaspar, D. A., Gomide, V. & Monteiro, F. J. The role of perfusion bioreactors in bone tissue engineering. Biomatter 2, 167–175 (2012).
9. Sacolick, L. I., Wiesinger, F., Hancu, I. & Vogel, M. W. B1 mapping by Bloch-Siegert shift. Magn. Reson. Med. 63, 1315–1322 (2010).
Figures