3121
A sample temperature control system for post mortem MRI
Sebastian Walter Rieger1, Karla Miller1, Peter Jezzard1, and Wenchuan Wu1
1Wellcome Centre for Integrative Neuroimaging, University of Oxford, Oxford, United Kingdom
1Wellcome Centre for Integrative Neuroimaging, University of Oxford, Oxford, United Kingdom
Synopsis
In post mortem MRI, the absence of body temperature autoregulation can lead to significant local heating in the sample from RF absorption and heat from the environment. This can interfere with scanning (such as diffusion measurements) and in unfixed samples, accelerate tissue decomposition. In this work, a temperature control system is presented which enables prolonged scanning of post mortem samples at a stable temperature while preserving tissue.
Introduction
During MRI scans, RF energy transmitted by the excitation pulses is absorbed by the body and causes tissue heating. In vivo, the heat is dissipated by the body’s thermoregulatory mechanisms (e.g. blood vessel dilation, perfusion), thus mitigating localised heating effects and regulating overall body temperature. However, in post mortem MRI, the absence of thermoregulation may lead to a substantial local temperature increase, particularly during long scans and at high SAR. In addition, where there is a difference between sample and room temperature, this will exacerbate the problem of temperature-related drift (preventing, for example, accurate quantitative diffusion imaging), and in the case of unfixed samples, may lead to accelerated tissue decomposition. The need for temperature control became apparent during investigative scanning in an unfixed porcine brain at 7T while monitoring sample and surrounding air temperature with fibre-optic temperature probes (T1S-10-W05-PT05, Neoptix, Canada). This was carried out in order to determine if tissue heating, whether by heat from the surroundings or by RF absorption, was likely to be of concern for accuracy of diffusion measurements and for preservation of unfixed tissue. Pre temperature control, the sample temperature rose by 5°C, and air temperature inside the coil by 2°C during a six hour scan (fig. 1). At the end of the scan, the temperature was still rising, suggesting that thermal equilibrium would not be reached in an acceptable timeframe and that the sample temperature needed to be controlled to slow down tissue decomposition during unfixed post mortem scans. In this work, a temperature control system was developed which allowed long post mortem scans (10 hours and more) to take place under conditions where energy deposition leads to tissue heating in the absence of autoregulation, while eliminating temperature-related signal drift and mitigating tissue decomposition in unfixed samples.Methods
The temperature control system (fig. 2) employed a thermal pad (Plastipad Infant CSZ-193, Cincinnati Sub-Zero, USA) wrapped around the sample, and connected to a recirculating cooler (F250, JULABO GmbH, Germany) using insulated flexible tubing. Cotton fabric was then wrapped around the outside of the pad to prevent excessive formation of condensation when the pad temperature was below ambient. The arrangement of male and female non-spill valved hose couplings (type NS4D220-06 (male) and NS4D170-06 (female), Colder Products Company, USA) allowed the chiller and circuit to be operated without the pad connected (fig. 2a), and so the circuit could be pre-cooled while the pad was being used for sample preparation. Once the circuit was at the desired temperature and the sample was ready for scanning, the pad was connected to the circuit (fig. 2b). The system was filled with deionised water, doped with manganese chloride at 5mM concentration in order to suppress MR signal. The system was first tested using a tissue mimicking phantom made from 500g of ground pork, shaped into a cylinder, with one temperature probe placed on the surface and the other in the centre. This allowed monitoring of heating effects both from RF absorption (heat generated inside the phantom) and convective and radiative heating from the surroundings (gradient and RF coils). The chiller set point was 4°C. The setup was subsequently deployed during two in situ, post mortem infant brain MRI scans, which included 3 hours of calibration and structural acquisitions followed by a 7 hour spin-echo diffusion protocol. In the first infant scan, this was preceded by additional sequence testing over one hour. The temperature probes were placed on the forehead and in the axilla, and the infant then wrapped in a layer of gauze, followed by the cooling pad, and an outer layer of cotton to prevent excessive condensation from forming on the outside of the pad. The chiller was set to 6°C for the first scan, and 8°C for the second.Results
Even though the tissue mimicking phantom had nearly warmed up to room temperature during preparation, its surface and internal temperature dropped to within a degree of the chiller setpoint within 2 to 3 hours (surface and centre, respectively). Temperatures were then stable to within ±0.4°C during the remaining structural and quantitative parameter mapping protocols, and a 7 hour diffusion scan (fig. 3a). In both post mortem infant scans, the sample temperature dropped and stabilised during the first 3 hours of setup and structural scanning, and then remained stable to within ±0.3°C throughout a 7 hour diffusion scan (fig. 3b/c). Example T2-, and diffusion weighted images are shown in fig. 4.Conclusion
The system provided reliable temperature control, even during prolonged scanning sessions, and adequate refrigeration for tissue preservation. It is therefore recommended that such a system of temperature control is used for post mortem scanning, in particular where temperature changes would interfere with the imaging process and in unfixed samples which are vulnerable to decomposition.Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union Seventh Framework Programme (FP/2007-2013)/ERC Grant Agreement no. 319456. We are grateful to the families who generously supported this trial. The Wellcome Centre for Integrative Neuroimaging is supported by core funding from the Wellcome Trust (203139/Z/16/Z). WW is supported by the Royal Academy of Engineering (RF\201819\18\92). KM is supported by the Wellcome Trust (WT202788/Z/16/A).References
No reference found.Figures
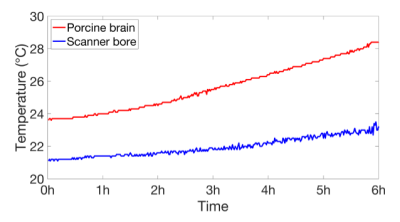
Temperature of unfixed porcine brain (red) and
ambient (blue) during a six-hour diffusion scan without active temperature
control.
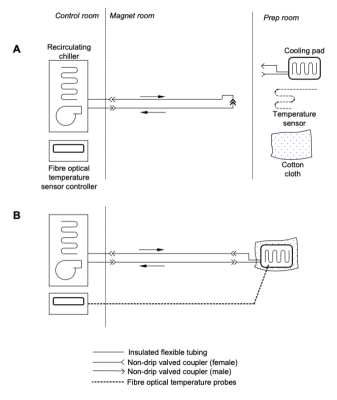
Cooling
system schematic. A: Pre-cooling / setup configuration - The chiller is
operating and pre-cooling the bulk of the fluid to the desired temperature,
while the cooling pad and temperature sensor(s) are being used for sample
preparation. B: Normal operation configuration - The temperature probe(s)
are affixed to the sample, and the cooling pad is wrapped around it. A layer of
cloth around the outside provides thermal insulation to prevent excessive
condensation, and the pad is connected to the chiller.
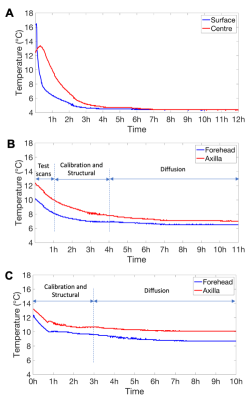
Sample
temperature during scanning with active temperature control. A: Core
(red) and surface (blue) temperature of the tissue mimicking phantom during
scanning, with active cooling (setpoint 4°C). B and C: Temperature
recorded during the two in situ post mortem infant scans, with active
cooling. Temperature probes were placed on the forehead (blue) and in the axilla
(red). Cooling system setpoints were 6°C (B) and 8°C (C).
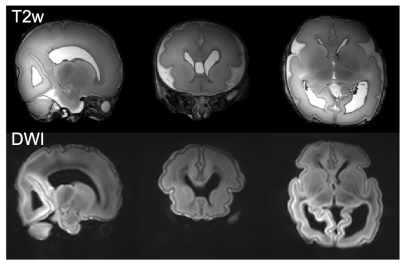
Example T2- and diffusion
weighted images. Top: T2 weighted images were acquired
at 0.4mm isotropic resolution using a 3D-SPACE sequence with FOV=150x150x96mm3,
TR/TE=2300/586ms, 6 repetitions. Bottom: averaged diffusion weighting images
acquired at 0.8mm isotropic resolution using a spin-echo diffusion weighted
readout-segmented EPI sequence with FOV=150x150x104mm3,
TR/TE=10.8s/113ms, multiband factor 2, GRAPPA factor 2, b=9000 s/mm2.