3113
Hardware developed for phase and frequency locking of interleaved MRI and DMI studies1Yale University, New Haven, CT, United States
Synopsis
Deuterium metabolic imaging (DMI) is a powerful new method to supplement the anatomical and functional information of MRI with a metabolic component. A reduction in overall scan time by interleaving MRI and DMI is clinically relevant, whereas research applications can benefit from complimentary information obtained through interleaved acquisition. Because most MR scanners only allow acquisition of one frequency, additional hardware is required to enable interleaved acquisition. Here we present hardware for the upconversion of 2H data into the 1H receive path and present a solution to achieve effective phase and frequency locking.
Introduction
Recent reports on the successful use of deuterium metabolic imaging (DMI) and 2H MRS in both animal models and patients have fueled a strong interest in these techniques for a range of applications.1-4 To complement the metabolic information of DMI with anatomical and functional MR images, it would be desirable to acquire DMI and MRI interleaved within the same experiment without extra scan time. Excitation of the 2H signal is typically achieved through the second, or decoupling channel. However, most MR scanners do not provide the means for interleaved acquisition of 1H and X nuclei signals. Hardware will be presented to overcome this problem by upconverting the 2H signal to the 1H frequency and acquiring all data as a 1H experiment. The hardware consists of an RF mixing stage with an RF switch, in addition to a phase and frequency agile direct digital synthesized based Local Oscillator (LO). The ability to modulate the starting phase of the LO, scan-to-scan, allows the DMI data to be averaged without need for post processing. Here we present data from phantoms and human brain, acquired with the new setup that facilitates interleaved DMI and MRI.Method
All data were acquired on a Bruker 4T 94cm Medspec scanner (Bruker Corporation. Ettlingen, Germany) using a single channel quadrature 1H volume coil with a 4 channel Tx/Rx 2H coil array. The new hardware consists of two assemblies; an RF mixer plus RF switch, and a LO controller. Excitation of the 2H nuclei is performed using the scanner’s decoupling channel. Real time control of the acquisition is from digital control lines from the scanner’s pulse program (PPG). Figure 1A shows the RF mixer block diagram. The 1H signal at 170 MHz is sent directly to the RF switch from the 1H preamplifier. The 2H signal at 26MHz is mixed with a LO frequency of 144 MHz to create a 170MHz 2H signal. The unwanted sideband of the mixing is suppressed with a filter and the signal is amplified before being sent to the RF switch. The switching for the correct RF path is defined by the PPG. Figure 1B shows the completed four channel RF mixer. The frequency and phase agile LO controller’s block diagram is illustrated in Figure 2A. Phase and frequency lists are sent from a networked PC and stored on the LO controller prior to the experiment. For each 2H acquisition, the phase and frequency are loaded at a precise time defined by the PPG using a digital control line. Using a preloaded offset list gives the LO the ability to not only lock the LO phase to the acquisition, but to also follow any phase and frequency pattern generated by the scanner. The LO signal is gated and only present for the 2H portion of the acquisition to prevent interference during the 1H experiment. Lastly, the small signal LO is filtered and amplified to the appropriate level to drive the RF mixer.Results
Figure 3 shows the phase variation during 2H MRS interleaved with 7-slice spin echo MRI. When unlocked (Figure 3A&B), a linear phase progression occurs over time due to the difference in frequency between the scanner’s receiver LO and that of the mixer’s LO as previously reported.5 In addition to the large linear phase drift, discrete consistent phase jumps appeared, which correlated with the DMI acquisitions during each repetition. These phase jumps were likely a propriety phase cycling used by the scanner to eliminate artifacts within its digital receiver. These jumps were consistent for a given pulse sequence and could be measured and compensated. Setting the phase of the LO to a consistent value prior to each acquisition eliminated the large linear phase drift. Adding additional precalculated phase offsets for each scan eliminated both the linear and discrete phase jump errors (Figure 3B&C). Interleaved 2H DMS signal from human brain (Figure 4A) was compared to direct 2H DMS (Figure 4B) to ensure that there is no loss of sensitivity using this method. The peak height SNR was found to be essentially identical. A comparison of interleaved DMI and FLAIR MRI (Figure 4C) with direct DMI (Figure 4C) in a phantom again resulted in identical sensitivity.Discussion
We have shown that it is possible to acquire DMI data within a 1H MRI experiment with no loss of sensitivity. Without correction it would not be possible to average or reconstruct DMI data directly without elaborate post processing. Our approach is to match the starting phase of the 2H data, scan to scan, by locking and tracking the phase in real time. We used a single channel 1H coil with four 2H coils as a proof of principle. The system is scalable and will ultimately be used with an eight channel 1H/2H coil. We have applied this hardware for an interleaved 2H acquisition but it is applicable for other nuclei, and on other hardware platforms provided they have a 10 MHz system reference and PPG digital control lines. On clinical systems, the LO can also be used as an RF source for interleaved acquisitions,5 or when acquiring non supported nuclei.6Acknowledgements
This research was funded, in part, by NIH grant NIBIB R01-EB025840.References
1. Lu, Ming, Zhu, Xiao-Hong, Zhang, Yi, Mateescu, Gheorghe, Chen, Wei. Quantitative assessment of brain glucose metabolic rates using in vivo deuterium magnetic resonance spectroscopy. J. Cereb. Blood Flow Metab. 2017;37:3518–3530 doi: 10.1177/0271678X17706444.
2. De Feyter HM, Behar KL, Corbin ZA, et al. Deuterium metabolic imaging (DMI) for MRI-based 3D mapping of metabolism in vivo. Sci. Adv. 2018;4:eaat7314 doi: 10.1126/sciadv.aat7314.
3. Kreis F, Wright AJ, Hesse F, Fala M, Hu D, Brindle KM. Measuring Tumor Glycolytic Flux in Vivo by Using Fast Deuterium MRI. Radiology 2019;294:289–296 doi: 10.1148/radiol.2019191242.
4. Riis-Vestergaard MJ, Laustsen C, Mariager CØ, Schulte RF, Pedersen SB, Richelsen B. Glucose metabolism in brown adipose tissue determined by deuterium metabolic imaging in rats. International Journal of Obesity 2020:1–11 doi: 10.1038/s41366-020-0533-7.
5. Niess F, Schmid AI, Bogner W, Wolzt M, Carlier P, Trattnig S, Moser E, Meyerspeer M. Interleaved 31 P MRS/1 H ASL for analysis of metabolic and functional heterogeneity along human lower leg muscles at 7T. Magn Reson Med. 2020 Jun;83(6):1909-1919. doi: 10.1002/mrm.28088. Epub 2019 Dec 17. PMID: 31846116; PMCID: PMC7065182.
6. Umathum R, Rösler MB, Nagel AM. In vivo 39K MR imaging of human muscle and brain. Radiology. 2013 Nov;269(2):569-76. doi: 10.1148/radiol.13130757. Epub 2013 Jul 22. PMID: 23878285.
Figures
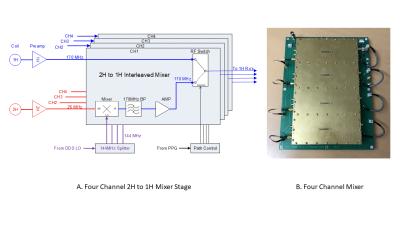
Figure 1 – (A) Four channel interleaved mixer. Inputs for the mixer are from their respective preamplifiers. The 1H signal is applied to one arm of the RF switch. The 2H signal is sent to an RF mixer and upconverted to 170MHz by mixing with the 144MHz LO. The upconverted signal is filtered to remove the unwanted sideband, amplified and sent to the RF switch. The PPG controls when each nucleus is sent to the scanner’s 1H receiver. (B) Each channel is isolated from outside interference and each other by using a dedicated RF shielded enclosure built onto the board.
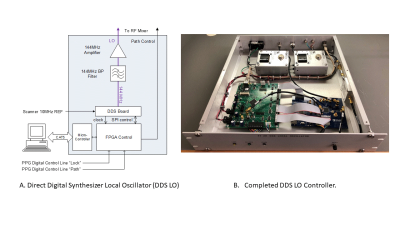
Figure 2 – (A) DDS LO controller containing two boards: An AD9910 Direct Digital Synthesizer (DDS) evaluation board (Analog Devices. MA, USA) to generate the RF and an FPGA board to control the DDS board via a networked PC. The scanner’s 10 MHz clock is multiplied to 640MHz and is used as a system clock by both boards ensuring all events are synchronized to the scanner. Phase and frequency information, from a preloaded onboard memory, is applied precisely under PPG control. The LO signal is filtered and amplified for optimal S/N and sent to the RF mixer (B) The completed controller.
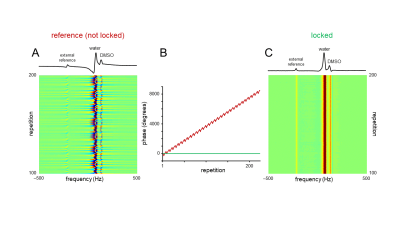
Figure 3 – Phase variation during 2H MRS interleaved with a 7-slice spin-echo MRI. The phantom consisted of three bottles containing ~0.1% D2O and various amounts of DMSO-D6 (~0.02 – 0.05%). A small (0.5 mL) phantom containing pure D2O served as an external off-resonance reference signal. (A) Phase variation in the absence of a phase lock. (B) In addition to the strong linear phase roll there is also a smaller sequence-dependent phase modulation. (C) In the presence of a phase lock, each spectrum has an identical phase. The 1D spectra shown in (A) and (C) are extracted from repetition 200.
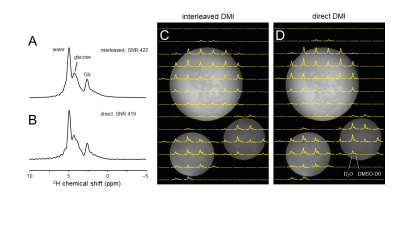
Figure 4 – (A) Interleaved and (B) direct 2H DMS signal from human brain acquired 60 min following oral [6,6’-2H2]-glucose administration. The peak height SNR in (A) and (B) is essentially identical. (C) Interleaved DMI and FLAIR MRI and (D) direct DMI of a phantom containing ~0.1% D2O and various amounts of DMSO-D6 (~0.02 – 0.05%) acquired as a 13 x 9 x 11 matrix over a 260 x 180 x 220 mm FOV. The information content, resolution and sensitivity of both datasets are essentially identical.