3096
Approaching order of magnitude increase of gradient strength: Non-linear breast gradient coil for diffusion encoding1Department of Radiology, Medical Physics, University Freiburg, Faculty of Medicin, Freiburg, Germany, 2Medical Physics in Radiology, German Cancer Research Center, Heidelberg, Germany, 3Department of Radiology, MR Physics, University Medical Center Erlangen, Erlangen, Germany, 4Department of Radiology, University Medical Center Erlangen, Erlangen, Germany, 5Department of Radiology, A. A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 6Harvard Medical School, Boston, MA, United States, 7Computer Assisted Clinical Medicine, Heidelberg University, Medical Faculty Mannheim, Heidelberg, Germany, 8High Field MR Center Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria
Synopsis
Local gradient coils allow for enhanced performance in terms of both gradient amplitude and switching rate. We recently presented the concept of a single channel non-linear breast gradient coil. Here we show results of the characterization, safety assessment and initial diffusion encoded images from phantom experiments of the prototype coil. Is an order of magnitude increase of gradient strength for diffusion weighting feasible using local gradient coils?
Introduction
Due to their compactness local gradient coils allow for enhanced performance in terms of both gradient amplitude, switching rate and PNS performance. Therefore, a renaissance of head-only gradient (insert) coils could be noticed in the past years [1,2]. The female human breast is another well-suited target for a dedicated gradient coil due to the fact that it can be partially enclosed [3-5]. Relaxing the constraint of linearity enables a further increase in gradient amplitude [6,7]. Therefore we chose to optimize one gradient channel for maximum gradient amplitude. It has been reported that diffusion anisotropy is significantly lower in breast cancers than in normal tissue [8]. However, an optimization for strongest amplitudes poses the problem of large variations of gradients over the target requiring additional acquisition and post processing steps and strategies. Our design was therefore optimized for linearity (= constant) gradient within coronal slices regardless of the gradient orientation [7]. We recently presented the concept of a single channel non-linear breast gradient coil [3]. One final question remains open: Is in vivo imaging feasible, using a non-linear gradient coil which allows for an order of magnitude increase of gradient strength for diffusion weighting?Here we present results of the characterization and safety assessment of the coil. Initial diffusion encoded images from phantom experiments are shown.
Methods
A dedicated non-linear breast (Fig.5) coil was developed in Freiburg which was manually wound using litz wire, assembled and cast under vacuum in-house. All measurements were performed on a 3T system, (Siemens Healthineers, Erlangen, Germany) equipped with additional gradient power amplifiers (GPAs) (IECO, Helsinki, Finland). Additional GPAs are controlled by custom interface hardware [9]. A dedicated transceiver coil consisting of a three loop solenoid was built to fit into the gradient coil. All pulse sequences were programmed in the open source pulse sequence framework PulSeq [10,11].Results
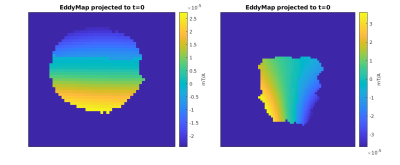
Measured Spatial Encoding Magnetic Field (SEM) maps (Fig.1 left) are in close agreement with simulated ones. Mean gradient of the measured maps depicts constant gradients within coronal slices while varying along the y-axis (Fig.1 right). A maximum sensitivity > 5mT/m/A in proximal parts of the breast and a mean sensitivity of 2.4mT/m/A is achieved.The shape of eddy current induced fields (Fig.2) closely correlates with the original fields, making a pre-emphasis feasible for eddy current correction. In agreement with simulations, the amplitude of eddy current induced fields was four orders of magnitude smaller than the original fields with decay times of 0.12s and 0.2s using a bi-exponential fit model.
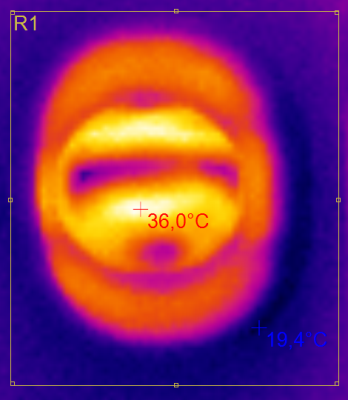
Heating from thermal losses inside the coil is limited by patient safety aspects and could potentially destroy the coil, if not controlled. Depicted in Fig.3 are thermal tests with 100A, a rather high duty cycle of 25% for 10 min without the RF coil resulted in a maximum surface temperature of 36°C. Simulations suggest the possibility for a safe operation of much higher currents. One should keep in mind that thermals measurements were performed without the RF-coil which acts as an additional thermal shield.
The sequence dependent acoustic pressure levels were measured for different diffusion sequence parameters up to 150 A and stayed below maximum peak values of 90 dB (unweighted). According to IEC standards, non-averaged and unweighted pressure levels are limited to 140 dB. A-weighted pressure levels for sequences are limited to a mean value of 99 dB(A) leaving a safe margin to further increase the current amplitude.
Detailed simulations of nerve stimulation thresholds using realistic body models to account for the directional dependence of nerve fibers were performed [12-14]. PNS is to be expected to occur for currents above 1400 A for the very short rise time of 0.1 ms while the threshold being in the order of 2500 A for rise times >0.5 ms. Electric fields in the heart region were simulated to be below 3 V/m for a current of 1000 A and 1 kHz switching rate. This is more than a factor two below the conservative cardiac stimulation IEC limit of 19.9 V/m at this frequency.
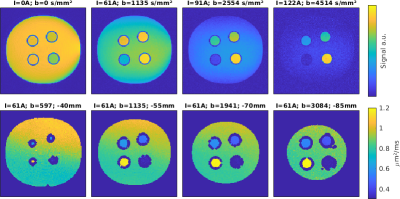
In Fig.4, the results of first diffusion weighted imaging experiments can be observed using a phantom filled with a polyvinylpyrrolidone (PVP K30) solution and containing additional tubes with different PVP concentrations in the range of 20% – 50 %. For high b-values, signal is only visible for high PVP concentrations, as expected. An initial comparison to theoretical ADC values based on the roughly estimated phantom temperature yielded deviations below 10%. The ratio of the ADC values meet the expectations with deviations < 5%.
Discussion
Deploying a GPA with a maximum current of 650A which is available at our facility in Freiburg will enable for a gradient strength exceeding 3T/m in proximal parts of the breast. However, due to the current corona virus situation a necessary controller board couldn't be installed.All safety relevant aspects such as PNS, thermal and acoustic emissions do not prohibit the use of a non-linear local breast gradient coil. Therefore an order of magnitude increase in gradient amplitude is feasible for diffusion weighting in the female human breast in vivo.
Acknowledgements
Deutsche Forschungsgemeinschaft (DFG) - Project number ZA 422/5-1References
[1] SK Lee MRM 2016 76:1939-50.
[2] M Weiger MRM 2018 79:3256-66.
[3] SA Maier, MRM 1998;39:392–401.
[4] SM Moon, MRM 2011;65:863–872.
[5] HS Lopez, JMR 2009;199:48 –55.
[6] F Jia, JMR
2017;281:217-228.
[7] F Jia, Proc. ISMRM 2020;4246.
[8] C Savannah, JMRI 2010;31(2):339-347.
[9] H Yu, Proc. ISMRM 2016;3548.
[10] KJ Layton, MRM 2016;77:1544–1552.
[11] http://pulseq.github.io/
[12] V Klein , MRM 2020;21
[13] M Davids, MRM 2019;81(1):686-701.
[14] M Davids, SciRep 2017;7(1):5316.
Figures
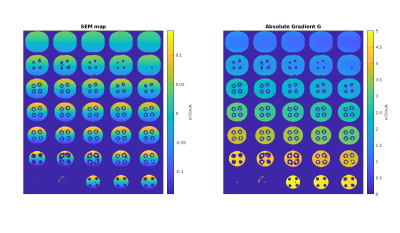
Fig 1: Measured spatial encoding magnetic field (SEM) maps (left) closely correlate with optimized and simulated ones. Gradient values (right) exhibit constant behavior within coronal slices. Gradient strength was optimized to be constant within coronal slices to reduce additional acquisition and post processing steps and strategies.