3078
Joint-CAIPI reconstruction of multi-echo GRASE data for fast, high-resolution myelin water imaging
Gian Franco Piredda1,2,3, Tom Hilbert1,2,3, Berkin Bilgic4,5,6, Erick Jorge Canales-Rodríguez3, Marco Pizzolato3,7, Reto Meuli2, Jean-Philippe Thiran2,3, and Tobias Kober1,2,3
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 2Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 3LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 4Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 5Department of Radiology, Harvard Medical School, Boston, MA, United States, 6Harvard‐MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 7Department of Applied Mathematics and Computer Science, Technical University of Denmark, Kongens Lyngby, Denmark
1Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, 2Department of Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 3LTS5, École Polytechnique Fédérale de Lausanne (EPFL), Lausanne, Switzerland, 4Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 5Department of Radiology, Harvard Medical School, Boston, MA, United States, 6Harvard‐MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 7Department of Applied Mathematics and Computer Science, Technical University of Denmark, Kongens Lyngby, Denmark
Synopsis
In previous work, CAIPIRINHA was implemented in multi-echo gradient and spin echo (GRASE) acquisitions for 3D myelin water imaging, achieving significant acceleration. However, residual undersampling artifacts prevented further acquisition time reduction. In this work, we propose a joint-CAIPI reconstruction across echoes of GRASE k-space data to remove aliasing artifacts and further accelerate the acquisition. Exploiting the redundant anatomical information across the different GRASE echoes helped mitigate aliasing artifacts in myelin water fraction maps and provided an additional ~40% reduction in scan time to 6:18$$$\,$$$minutes for a whole-brain acquisition at 1.6$$$\,$$$mm3 isotropic resolution.
Introduction
Myelin water imaging techniques based on T2 relaxometry proved to deliver quantitative measurements specific to myelin alterations1–4. Recently, image resolution and acquisition time (TA) to obtain 3D whole-brain multi-echo T2 data could be improved by combining parallel imaging with the multi-echo gradient and spin echo (GRASE) sequence5. However, using standard encoding and reconstruction techniques resulted in residual artifacts at high undersampling rates, and precluded further TA reduction without compromising image quality.Following recent advances in the reconstruction of MR acquisitions with multiple echoes/phase-cycles/contrasts6,7, this work proposes to exploit controlled aliasing, complementary encoding and redundant anatomical information across the different GRASE echoes in a joint-CAIPI (J-CAIPI) reconstruction to improve image quality and possibly further reduce the TA.
Methods
Sampling schemesTo investigate joint reconstruction of multi-echo GRASE data while preserving the contrast information in each echo, autocalibration signals (ACS) need to be acquired in the k-space of each echo. Therefore, in contrast to the previously proposed CAIPI undersampling5 (Figure 1A), a sampling pattern comprising an integrated ACS region and CAIPI undersampling of the outer k-space on an elliptical Cartesian grid was implemented (Figure 1B). Additionally, the implementation allows shifting the sampling patterns across echoes in both phase encoding directions, providing a complementary k-space coverage across the echoes which proved to enhance the output of joint reconstructions6 (Figure 1C). We also propose and investigate a further possibility to accelerate the scan time by retrospectively undersampling the ACS region (Figure 1D-E).
Reconstruction methods
The reference CAIPI acquisitions were reconstructed after calibrating 3D convolution kernels on an external scan (i.e. a fast ACS scan of ~3.5$$$\,$$$seconds performed before the actual GRASE acquisition).
Undersampled data with integrated ACS were reconstructed both with CAIPI, calibrating the 3D convolution kernels on the ACS region, and with a J-CAIPI algorithm that extends previous work on joint-GRAPPA6. In J-CAIPI, k-space data are first concatenated along the echo dimension to create additional channels as extra coils and then reconstructed using 3D CAIPI convolutional kernels. The number of available coils was compressed8 from 52 to eight for faster reconstruction in all experiments.
A significant amount of acquisition time is spent to sample the ACS region in integrated scans. Hence, to explore further acceleration possibilities, ACS regions were retrospectively undersampled and a two-step reconstruction was investigated: first, the integrated ACS regions were reconstructed using kernels trained on an external ACS scan; subsequently, both CAIPI and J-CAIPI algorithms were used to reconstruct the echo images.
Dataset
Three healthy volunteers (all male, [27-35]$$$\,$$$y/o) were scanned using a prototype 3D multi-echo GRASE sequence (whole-brain, 1.6$$$\,$$$mm3 isotropic) using three different sampling schemes: 1) external reference scan (Ny$$$\times$$$Nz$$$\,$$$=$$$\,$$$32$$$\times$$$32) and CAIPI Ry$$$\times$$$RzΔkz$$$\,$$$=$$$\,$$$3$$$\times$$$2(1) (TA$$$\,$$$=$$$\,$$$10:30$$$\,$$$minutes); 2) integrated ACS (Ny$$$\times$$$Nz$$$\,$$$=$$$\,$$$32$$$\times$$$32) and CAIPI Ry$$$\times$$$RzΔkz$$$\,$$$=$$$\,$$$3$$$\times$$$3(1), without and 3) with complementary k-space shifts across echoes (TA$$$\,$$$=$$$\,$$$10:34$$$\,$$$minutes). A 3D MPRAGE image was also acquired as anatomical reference. All sequence parameters are detailed in Table 1. The two acquisitions with integrated ACS were retrospectively undersampled following a CAIPI Ry$$$\times$$$RzΔkz$$$\,$$$=$$$\,$$$2$$$\times$$$2(1) scheme, which would correspond to an effective TA of 6:18$$$\,$$$minutes.
Myelin water fraction comparison
Myelin water fraction (MWF) maps were computed from the multi-echo T2 volumes using a non-negative least-square optimization with second-order Tikhonov regularization9. Averaged MWF values in white matter regions were extracted from segmentation masks automatically computed on the MPRAGE contrast10, and compared via Bland-Altman analysis.
Results
Residual aliasing artifacts in the T2-weighted images were found to be more prominent in the data with higher acceleration rates and to be located at different positions in the shifted k-space acquisitions (Figure 2). Thanks to this complementary sampling, artifacts in shifted k-space data were drastically mitigated in the J-CAIPI reconstructions (Figure 2).The residual artifacts in CAIPI reconstructions with higher acceleration rate in the outer k-space were found to severely compromise the image quality of the retrieved MWF maps (Figure 3). Conversely, such artifacts were cancelled out in MWF maps obtained from the J-CAIPI reconstruction of shifted k-space data (Figure 3). These maps also showed a more homogenous contrast over brain regions, as shown for instance by the MWF standard deviation in the corpus callosum, which was found to decrease from 5.97% in conventional CAIPI to 3.46% in J-CAIPI (Figure 3). Average MWF values across the enrolled volunteers are shown in Figure 4A. The Bland-Altman analysis comparing the MWF values obtained from J-CAIPI reconstructed k-space with fully sampled and retrospectively undersampled ACS regions revealed a mean bias of 0.38% with the limits of agreement spanning from -1.80% to 0.99% (Figure 4C).
Discussion and Conclusion
In this work, the J-CAIPI reconstruction of multi-echo GRASE data with integrated ACS and k-space shifts across echoes was proposed as an alternative to recently proposed CAIPI schemes5. While preserving comparable MWF values, J-CAIPI reconstructed data were found to be free from aliasing artifacts. Additionally, we showed that the J-CAIPI reconstruction can work robustly from undersampled integrated ACS regions reconstructed from an external ACS scan, allowing to further reduce the TA from 10:30$$$\,$$$minutes to 6:18$$$\,$$$minutes for a 1.6$$$\,$$$mm3 isotropic whole-brain scan.Future work is planned to implement the prospective undersampling of the ACS region and the random sampling of the outer k-space to incorporate compressed sensing priors into J-CAIPI reconstruction, which showed good promise in single-echo GRASE imaging11.
Acknowledgements
No acknowledgement found.References
- Mackay A, Whittall K, Adler J, Li D, Paty D, Graeb D. In vivo visualization of myelin water in brain by magnetic resonance. Magn Reson Med. 1994;31(6):673-677. doi:10.1002/mrm.19103106143.
- Laule C, Leung E, Li DK, et al. Myelin water imaging in multiple sclerosis: quantitative correlations with histopathology. Mult Scler J. 2006;12(6):747-753. doi:10.1177/13524585060709284.
- Alonso-Ortiz E, Levesque IR, Pike GB. MRI-based myelin water imaging: A technical review. Magn Reson Med. 2015;73(1):70-81. doi:10.1002/mrm.251985.
- Piredda GF, Hilbert T, Thiran J, Kober T. Probing myelin content of the human brain with MRI: A review. Magn Reson Med. 2021;85(2):627-652. doi:10.1002/mrm.285092.
- Piredda GF, Hilbert T, Canales‐Rodríguez EJ, et al. Fast and high‐resolution myelin water imaging: Accelerating multi‐echo GRASE with CAIPIRINHA. Magn Reson Med. 2021;85(1):209-222. doi:10.1002/mrm.284276.
- Bilgic B, Kim TH, Liao C, et al. Improving parallel imaging by jointly reconstructing multi-contrast data. Magn Reson Med. 2018;80(2):619-632. doi:10.1002/mrm.270767.
- Bilgic B, Chatnuntawech I, Manhard MK, et al. Highly accelerated multishot echo planar imaging through synergistic machine learning and joint reconstruction. Magn Reson Med. 2019;82(4):1343-1358. doi:10.1002/mrm.278138.
- Zhang T, Pauly JM, Vasanawala SS, Lustig M. Coil compression for accelerated imaging with Cartesian sampling. Magn Reson Med. 2013;69(2):571-582. doi:10.1002/mrm.242679.
- Canales-Rodríguez EJ, Pizzolato M, Piredda GF, et al. Robust Myelin Water Imaging from multi-echo T2 data using second- order Tikhonov regularization with control points. In: Proceedings of the International Society of Magnetic Resonance in Medicine, Montreal, Canada. 2019. Abstract 4901.
- Schmitter D, Roche A, Maréchal B, et al. An evaluation of volume-based morphometry for prediction of mild cognitive impairment and Alzheimer’s disease. NeuroImage Clin. 2015;7:7-17. doi:10.1016/j.nicl.2014.11.00111.
- Cristobal-Huerta A, Poot DHJ, Vogel MW, Krestin GP, Hernandez-Tamames JA. Compressed Sensing 3D-GRASE for faster High-Resolution MRI. Magn Reson Med. 2019;82(3):984-999. doi:10.1002/mrm.27789
Figures
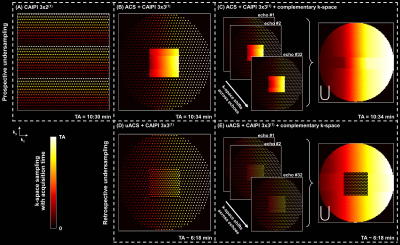
Figure 1. k-space sampling strategies investigated in this study. (A) CAIPI 3x2(1) (acquired with an external reference scan for calibrating the reconstruction kernels). (B) CAIPI 3x3(1) on an elliptical Cartesian grid with an integrated autocalibration signal (ACS) region and (C) with additional shifts across echoes for complementary k-space coverage. (D, E) Same as in (B) and (C), respectively, but with retrospective undersampling of the ACS region with a CAIPI 2x2(1) pattern. Acquisition times (TAs) are given for a 1.6 mm3 isotropic whole-brain scan.
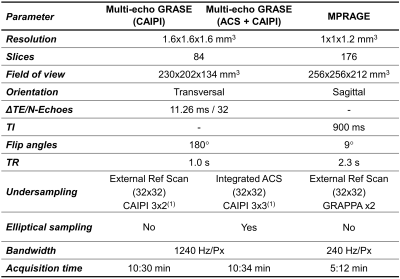
Table 1. Parameters of the acquired sequences. In each scanning session, the multi-echo GRASE sequence with integrated autocalibration signal (ACS) was acquired twice, respectively without and with complementary k-space sampling across echoes.
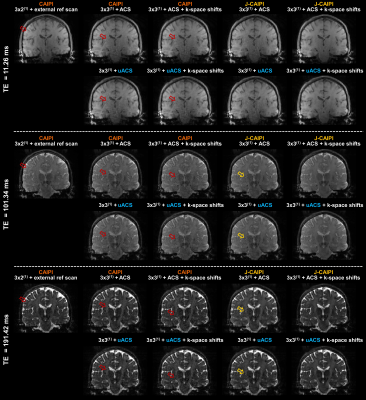
Figure 2. Three T2-weighted contrasts acquired with different k-space sampling schemes or with retrospective undersampling of the ACS region (uACS) and reconstructed with conventional and joint-CAIPI (J-CAIPI). Residual aliasing artifacts in CAIPI reconstructions (red arrows) are mitigated in J-CAIPI reconstructions but still present if k-space shifts across echoes are not employed (yellow arrows). Comparable image quality is appreciated between images reconstructed from fully sampled and retrospectively undersampled ACS regions.
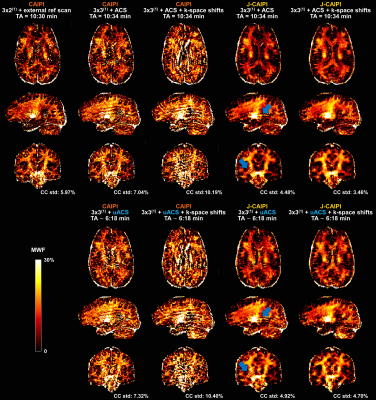
Figure 3. Example MWF maps obtained from T2-weighted contrasts acquired with different k-space sampling schemes or with retrospective undersampling of the ACS region (uACS) and reconstructed with conventional and joint-CAIPI (J-CAIPI). Residual aliasing artifacts in CAIPI reconstructions with integrated ACS heavily compromise image quality of retrieved MWF maps. Such artifacts are mitigated in J-CAIPI reconstructions but still present if k-space shifts across echoes are not employed (blue arrows). MWF std in corpus callosum (CC) decreases in J-CAIPI reconstructions.
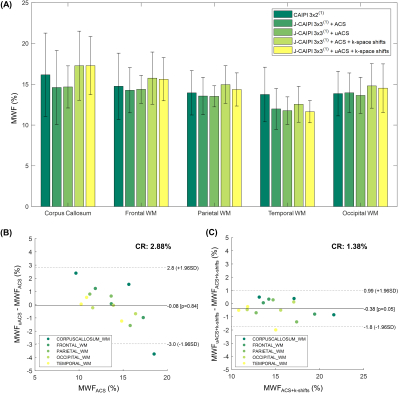
Figure 4. (A) Average MWF values in white matter (WM) regions across the three enrolled healthy volunteers. Error bars indicate one standard deviation. (B,C) Bland-Altman agreement plots reporting the difference between MWF obtained from k-space acquired with an integrated ACS region and from the corresponding retrospectively undersampled ACS region (all reconstructed with joint-CAIPI (J-CAIPI) and (B) without and (C) with k-space shifts across echoes).