3060
Characterisation of the flip angle dependence of R2* in Multi-Parameter Mapping1Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 2Centre de Résonance Magnétique des Systèmes Biologiques, UMR5536, CNRS/University Bordeaux, Bordeaux, France, 3Laboratory for Research in Neuroimaging, Department for Clinical Neuroscience, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4Department of Systems Neurosciences, University Medical Center Hamburg-Eppendorf, Hamburg, Germany, 5Department of Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany
Synopsis
The apparent transverse relaxation rate (R2*) has biologically-relevant dependence on iron content and myelination. However confounding factors, e.g. flip angle dependence owing to differential longitudinal relaxation rates of sub-compartments, hinder interpretation. Multi-compartment models have been used to estimate myelin-water fraction from multi-echo spoiled GRE images, but require rich datasets for reliable estimation leading to extended acquisition times. A time-efficient alternative is to assume mono-exponential intra-voxel decay. In this work, we characterise the residual FA-dependence of such R2* estimates in vivo, and explore the biological origin of this dependence via simulations.
Introduction
Estimates of the apparent transverse relaxation time (R2*) in the brain have biologically-relevant dependence on features such as iron content and myelination, and have been used to map age-related differences (1) as well as pathological change (2). However, confounding factors such as orientation (3) and flip angle (FA) dependence (4) complicate interpretation. The FA-dependence stems, at least in part, from the multi-compartment nature of the MRI signal, e.g. contributions from both myelin and intra-extracellular water environments. Multi-compartment models have been used to estimate myelin water fraction (fMW) from multi-echo spoiled gradient echo (SPGR) images but require extended echo trains that lengthen the acquisition time, limit spatial resolution and enhance sensitivity to physiological effects (5) and noise (6). Fitting highly parameterised models is challenging and benefits from a rich array of data for reliable estimation (7). To mitigate these challenges, neuroscientific studies commonly make the simplifying assumption of a mono-exponential decay to obtain a single R2* estimate per voxel using data that can be acquired in clinically feasible scan times (8). In this work, we quantify the residual FA-dependence of these estimates in vivo at 7T, and use simulations to explore the biological origin of this dependence.Methods
In vivo AcquisitionsA multi-parameter mapping (MPM) quantitative MRI protocol was extended to acquire multi-echo (TE=[2.56:2.30:14.5]ms) SPGR data at four nominal flip angles (FA=[6°, 9°, 26°, 42°]). TR was 19.5ms, FOV of 192×192×160mm3 with 1mm isotropic resolution, and spoiling was achieved with an RF spoiling increment of 144° and 6π spoiler moment per TR. Transmit field inhomogeneity (B1+eff) was measured using an in-house sequence exploiting the Bloch-Siegert shift (9), with relevant acquisition parameters including: TE/TR=6.77/40ms, 14° flip angle, FOV of 256×256×192mm3 with 4mm isotropic resolution, 90° RF spoiling increment.
R2* maps were estimated for each FA independently via a log-linear fit across echo times. A heuristic linear model, incorporating transmit field inhomogeneity, was applied to the R2* maps to partition the FA-independent (R2*′) and FA-dependent (β) components of the decay voxel-wise, i.e.:
R2*(FA) = R2*′ + β FA B1+eff (Eq. 1)
Simulations
Simulation settings matched those of the acquisitions. Two compartments (10), assumed to be a fast relaxing myelin-water compartment (T1=280ms, T2=T2*=8ms for simplicity) and a slower relaxing intra-extracellular compartment (T1=1700ms, T2=T2*=36ms), were simulated using the Bloch-McConnell equations implemented in the EPG formalism (11). Signal decay, according to the compartment-specific T2* was applied, before sampling the magnitude of the net signal at echo times matching the acquisitions. Myelin water fraction (fMW) was varied between 2% and 20% with a step size of 2% to simulate different tissues conditions (encompassing white mater (WM) fMW=16% and grey matter (GM) fMW = 6% (10)). Different exchange regimes were investigated by varying a directional (myelin to IE) residency time between 100ms and 500ms in steps of 100ms (7). The same heuristic linear model (Eq. 1) was fitted to each of these simulated datasets.
Results
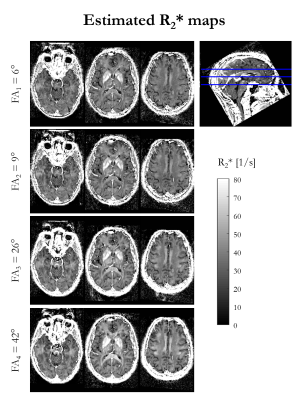
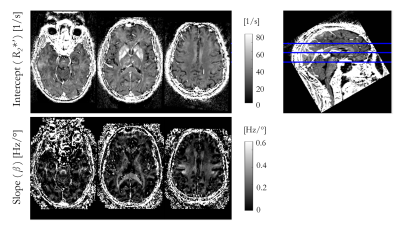
Figure 1 shows the R2* maps estimated for each of the four nominal FA. An increase in R2* is visually apparent with increasing flip angle, most notably in the corpus callosum.Figure 2 shows the result of fitting the heuristic linear model of R2* in vivo. The intercept (R2*′) captures the majority of the spatial variability and bears close resemblance to the FA-specific R2* maps. The spatial variability in the slope (β) highlights specific, predominantly white matter, structures, such as the superior cerebellar fibres, brachium conjunctivum and corpus callosum. The slope was negligible in iron-rich structures such as the putamen.
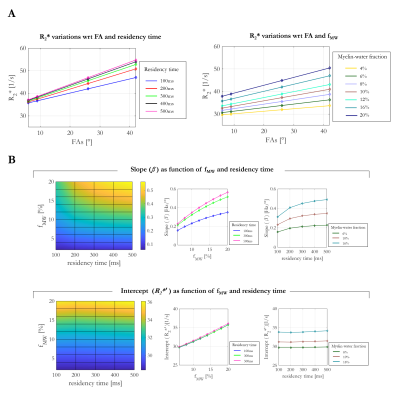
Simulations consistently showed increasing R2* estimates as a function of FA (Figure 3A) regardless of residency times or fMW. The linear dependence observed in vivo was recapitulated in the simulations. Figure 3B show the slope (β) and intercept (R2*′) obtained from a linear fit of the simulation-derived R2* estimates, as a function of fMW and residency time. The intercept was most sensitive to fMW (12.6%), but effectively independent of the residency time (0.74% variance across range investigated). The slope was also most sensitive to the fMW (~55%), but additionally depended on the residency time (~13%). The FA-dependent component of R2* (β) was largest in the case of long residency time and high fMW, and smallest when the residency time was short and fMW small.
Discussion
R2* estimates assuming a mono-exponential decay provide high quality maps with contrast driven by iron rich structure and distinct fibre pathways. Even with comparatively short echo times and few echoes, a dependence on FA was observed in vivo at 7T, indicative of multi-compartment intra-voxel relaxation. With a heuristic linear model of the FA-dependence, we observed excellent agreement between the empirical observations and simulations based on just two exchanging water pools (c.f. predicted versus measured values). The fitted offsets predicted by simulation are in line with those obtained in vivo in GM and WM. Higher R2* is observed in iron-rich structures not expected to be well represented by these simulations.The simulations indicate that the FA-dependence (β) is largest when fMW is appreciable and the exchange regime is slow, and is smallest when the fMW is low and exchange is fast.
Acknowledgements
The Wellcome Centre for Human Neuroimaging is supported by core funding from the Wellcome [203147/Z/16/Z].References
1. Callaghan MF, Freund P, Draganski B, et al. Widespread age-related differences in the human brain microstructure revealed by quantitative magnetic resonance imaging. Neurobiol. Aging [Internet] 2014;35:1862–1872. doi: 10.1016/j.neurobiolaging.2014.02.008.
2. Zhao Y, Raichle ME, Wen J, et al. In vivo detection of microstructural correlates of brain pathology in preclinical and early Alzheimer Disease with magnetic resonance imaging. Neuroimage [Internet] 2017;148:296–304. doi: 10.1016/j.neuroimage.2016.12.026.
3. Papazoglou S, Streubel T, Ashtarayeh M, et al. Biophysically motivated efficient estimation of the spatially isotropic component from a single gradient‐recalled echo measurement. Magn. Reson. Med. [Internet] 2019:mrm.27863. doi: 10.1002/mrm.27863.
4. Weiskopf N, Callaghan MF, Josephs O, Lutti A, Mohammadi S. Estimating the apparent transverse relaxation time (R2*) from images with different contrasts (ESTATICS) reduces motion artifacts. Front. Neurosci. [Internet] 2014;8:1–10. doi: 10.3389/fnins.2014.00278.
5. Nam Y, Kim DH, Lee J. Physiological noise compensation in gradient-echo myelin water imaging. Neuroimage [Internet] 2015;120:345–349. doi: 10.1016/j.neuroimage.2015.07.014.
6. Nam Y, Lee J, Hwang D, Kim D-H. Improved estimation of myelin water fraction using complex model fitting. Neuroimage [Internet] 2015;116:214–221. doi: 10.1016/j.neuroimage.2015.03.081.
7. Chan K-S, Marques J. Multi-compartment relaxometry and diffusion informed myelin water imaging – promises and challenges of new gradient echo myelin water imaging methods. Neuroimage 2020;221:117159. doi: 10.1016/j.neuroimage.2020.117159.
8. Tabelow K, Balteau E, Ashburner J, et al. hMRI – A toolbox for quantitative MRI in neuroscience and clinical research. Neuroimage [Internet] 2019;194:191–210. doi: 10.1016/j.neuroimage.2019.01.029. 9. Corbin N, Acosta‐Cabronero J, Malik SJ, Callaghan MF. Robust 3D Bloch‐Siegert based mapping using multi‐echo general linear modeling. Magn. Reson. Med. [Internet] 2019;82:2003–2015. doi: 10.1002/mrm.27851.
10. Labadie C, Lee J-H, Rooney WD, Jarchow S, Aubert-Frécon M, Springer CS, Möller HE. Myelin water mapping by spatially regularized longitudinal relaxographic imaging at high magnetic fields. Magn. Reson. Med. [Internet] 2014;71:375–87. doi: 10.1002/mrm.24670.
11. Malik SJ, Teixeira RPAG, Hajnal J V. Extended phase graph formalism for systems with magnetization transfer and exchange. Magn. Reson. Med. [Internet] 2018;80:767–779. doi: 10.1002/mrm.27040.
Figures