3046
Parallel transmission for variable flip angle T1 mapping at 7T: initial experiences1Department of Neurophysics, Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 2Siemens Healthcare GmbH, Erlangen, Germany, 3Wellcome Centre for Human Neuroimaging, UCL Queen Square Institute of Neurology, University College London, London, United Kingdom, 4Felix Bloch Institute for Solid State Physics, Faculty of Physics and Earth Sciences, Leipzig University, Leipzig, Germany
Synopsis
In this abstract we detail our first experiences using parallel transmission (pTx) to quantitatively map T1 via a dual flip-angle approach. We present data from a human volunteer scanned on the 7T Siemens MAGNETOM Terra using the pTx system and a Nova 32-channel receive / 8-channel transmit coil, with online optimized kT-points RF pulses. Data from the same coil in static CP mode (without pTx) was additionally acquired. With the demonstrated reduction in B1+ inhomogeneity, various bias correction schemes used at lower field strengths may become applicable.
Introduction
Ultra-high field (UHF) strength MRI enables quantitative mapping of the longitudinal relaxation rate (R1 = 1/T1) at sub-millimetre resolutions in vivo, in turn furthering our understanding of brain microstructure1. A drawback is the poorer RF excitation uniformity which significantly biases R1 estimates derived from dual flip angle measurements. Parallel transmission (pTx) offers the potential to improve B1+ homogeneity in the imaging volume and therefore to reduce spurious spatial variance in R1 estimates.We investigated whether exploiting pTx by replacing the excitation pulse with optimized kT-points RF pulses would reduce B1+ related biases in R1 estimates to a level that could be addressed by post-hoc correction schemes, which are widely used at lower field strengths to remove residual biases, and do not require additional scans to map the B1+ inhomogeneity.
Methods
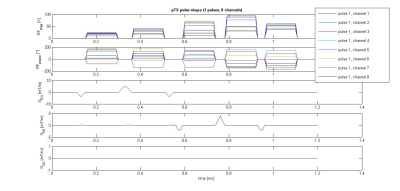
A healthy volunteer was scanned with a 7T MRI system (MAGNETOM Terra, Siemens Healthineers, Germany) equipped with a 8Tx/32Rx head RF coil (Nova Medical, Wilmington, USA). The RF system was operated in two frameworks: (a) with the coil transmit elements in a fixed magnitude/phase relationship (approximating CP mode) using conventional non-selective sinc-shaped excitation pulses with a bandwidth-time product of 6, and (b) with dynamic pTx pulses using the kT-points trajectory. Time and SAR constraints allowed 5 kT-points: one was placed in the centre of the excitation k-space and the position of the others was optimized on the kx-ky plane offline. The RF magnitude and phase were optimized running an online optimization directly before the main scan. The pulse optimization process runs without user intervention, suiting the practical workflow of a neuroimaging center. The online optimization including acquiring B1+ and B0 maps took less than two minutes. The 5 kT-points pulse with a flip angle of 23 degrees is shown in Figure 1.In each mode, an in-house built sequence was used to acquire 3D multi-echo FLASH images with both PD (nominal flip angle 8 degrees) and T1 (23 degrees) weightings. The other parameters were: 1 mm isotropic resolution (matrix size 224 x 256 x 176), TR 24.1 ms, eight evenly spaced gradient echoes (TE 2.5 .. 18.2 ms), GRAPPA acceleration factor 2 in both 2D and 3D phase encoding directions, gradient and RF spoiling applied, leading to an acquisition time per volume of 4.5 minutes.
Images were processed using the hMRI toolbox (http://hmri.info 2) within SPM (https://www.fil.ion.ucl.ac.uk/spm) and MATLAB (MathWorks, Natick, USA), modified to avoid the small-angle approximation, to first yield R1 maps without B1-correction. We then applied a unified-segmentation-based correction (UNICORT)3 which estimates B1+ inhomogeneity and produces corrected R1 maps without requiring measured B1+ field maps.
Results
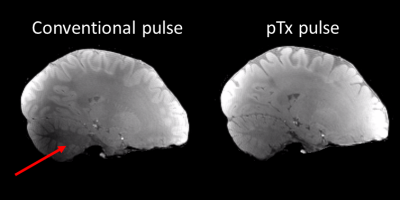
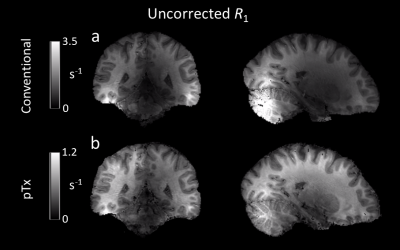
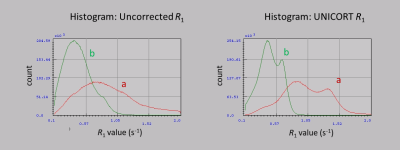
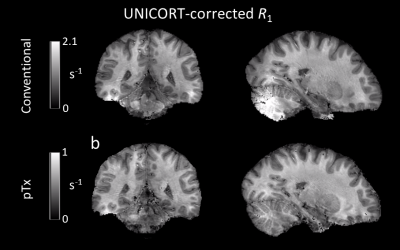
Without the application of optimized pTx pulses, signal dropouts were observed in weighted FLASH images (Figure 2) leading to severe artifacts in the uncorrected R1 maps (Figure 3, (a)), e.g., in the posterior cerebellum and some areas of the temporal lobes. Using the optimized pTx pulses led to a marked improvement in the artifact level (Figure 3, (b)) and to a reduction in the artifactual spread of R1 values. Within a white matter mask, the span of uncorrected R1 values (25th-75th quartile, Figure 4) decreased from 0.65 s-1 to 0.28 s-1, implying a substantial improvement in B1+ homogeneity. The combination of pTx with UNICORT (Figure 5) yielded rather uniform R1 maps, although R1 values were clearly underestimated.Discussion and Conclusions
B1+ inhomogeneities at 7T were reduced to the range where bias corrections are known to perform well at lower field strengths (<= 3T). In this first implementation, we employed a post-processing method (UNICORT) for reducing residual bias, since highly accurate and precise B1+ mapping methods required for quantitative mapping are still under investigation (flip angle errors enter R1 calculations quadratically3) and can lead to substantial error propagation when sub-optimal4. The UNICORT method does not account for global B1+ offsets and thus led to underestimation of R1, which may be addressed in the future by additional reference scans. Further areas of investigation are inadvertent MT effects5 and interaction with physiological noise.Acknowledgements
The research leading to these results has received funding from the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement no. 616905. The Wellcome Centre for Human Neuroimaging is supported by core funding from Wellcome [203147/Z/16/Z].References
1. Trampel R, Bazin P-L, Pine K, et al. In-vivo magnetic resonance imaging (MRI) of laminae in the human cortex. Neuroimage. 2019;197:707-715.
2. Tabelow K, Balteau E, Ashburner J, et al. hMRI – A toolbox for quantitative MRI in neuroscience and clinical research. Neuroimage. 2019;194:191-210.
3. Weiskopf N, Lutti A, Helms G, et al. Unified segmentation based correction of R1 brain maps for RF transmit field inhomogeneities (UNICORT). Neuroimage. 2011;54(3):2116-24.
4. Lee Y, Callaghan M, Nagy Z. Analysis of the Precision of Variable Flip Angle T1 Mapping with Emphasis on the Noise Propagated from RF Transmit Field Maps. Front. Neurosci. 2017;11:106.
5. Teixeira RP, Malik S, Hajnal J. Fast quantitative MRI using controlled saturation magnetization transfer. Mag. Reson. Med. 2019;81(2):907-920.
Figures