3042
Abnormal intrinsic brain functional network dynamics associated with tremor in Parkinson’s disease1Department of Medical Imaging, Peking University Shenzhen Hospital, Shenzhen, China
Synopsis
By applying group information guided independent component analysis to fMRI data, the dFNCs of each subject were estimated using a sliding window method and k-means clustering. Four dynamic functional states were identified. Interestingly, our study found that dwell time in State I was positively correlated with resting tremor scors in TD-PDs dFNC strength between BG and vSMN in State3 had significant positive correlation with MDS-UPDRS-III score in PD patients. Our study indicates that tremor in Parkinson’s disease is characterized by altered dFNC strength and temporal properties in dynamic connectivity, which provides a new insight into the pathological of Parkinsonian tremor.
Purpose
Parkinson’s disease (PD)is the second most common neurodegenerative disorder in the elderly after Alzheimer’s disease, characteristic by tremor,bradykinesia,rigidity and postural instability. Resting tremor is core diagnostic feature affecting up to 70% of Parkinson’s patients and a symptom relatively distinct from other motor signs of PD,less reliably responsive to dopaminergic modulation and does not worsen at the same rate as bradykinesia and rigidity.Previous studies have reported abnormalities in functional brain networks in Parkinson disease tremor, yet most of them were based on static functional connectivity with the assumption that brain intrinsic fluctuations throughout the entire scan are constant. However,the dynamic characteristics of brain networks associated with tremor in Parkinson’s disease remains poorly understood. Therefore, we intend to quantify the characteristics of dynamic functional connectivity (dFNC) in PD patients with resting tremor at subnetwork levels,mainly focus on the temporal properties of functional connectivity states using resting state functional magnetic resonance imaging (fMRI).Materials and methods
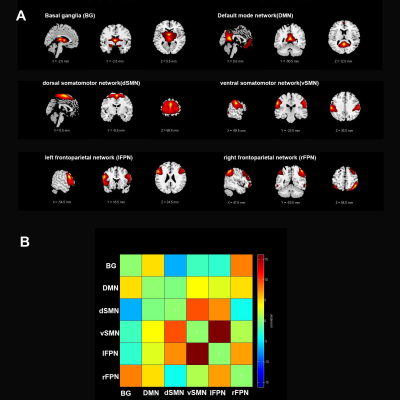
From the Parkinson’s Progression Marker Initiative database, 58 patients with PD (31 with resting tremor, TD-PD and 27 without resting tremor, NTD-PD) and 20 age and sex matched healthy controls (HC) were enrolled in our study. PD patients were divided into 2 groups based on the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) part III of resting tremor score. By applying independent component analysis(ICA) to fMRI data, selected from the 39 independent components (ICs), six intrinsic connectivity networks of interest were identified for subsequent analyses:basal ganglia (BG), default mode network (DMN), dorsal somatomotor network(dSMN), ventral somatomotor network (vSMN), left frontoparietal network (lFPN) and right frontoparietal network (rFPN).Furthermore, the dFNCs of each subject were computed utilizing a sliding window method, and then a k-means algorithm was used to cluster all dFNC windows,resulting in identification of four dynamic functional states that recurred throughout individual scans and across subjects.Next,three different variables in the state transition vector including fractional windows, mean dwell time and number of transitions were assessed to examine the temporal properties of dFNC states.Group comparisons were performed on functional connectivity strengths and temporal properties of FNC matrices followed by post hoc tests with false discovery rates-correction for multiple comparisons.Additionally,we performed Pearson’s correlations analyses between altered dynamic functional connectivity properties and clinical variables including resting tremor score, total tremor score as well as MDS-UPDRS-III score.Results
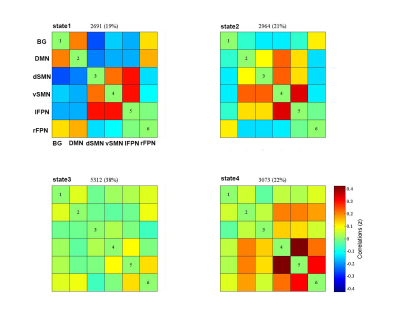
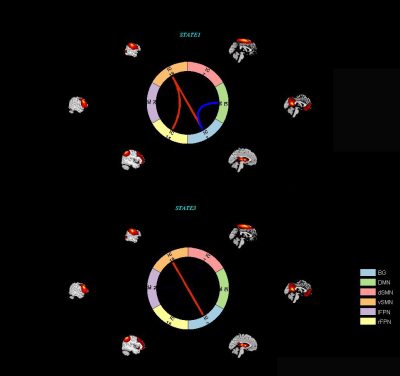
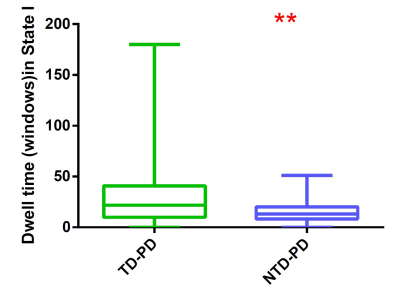
A k-means clustering algorithm identified four different dFNC states and the cluster centroid of each dFNC state is shown in figure 2. Among four dFNC states, State 3 was characterized by relatively weak connectivity, occurred most frequently in all states(38%), while State 1 was characterized by relatively strong positive and negative connectivity, occurred least frequently. Compared to PD patients without resting tremor, the mean dwell time in State I was significantly longer than the PD patients with resting tremor(FDR corrected, Figure 3) . Either fractional windows or number of transitions was not significantly different between groups. Compared to NTD-PDs, TD-PDs showed increased FNC strength between basal ganglia and ventral somatomotor in State 1 and State 3, and the connectivity between right frontoparietal and vSMN in State1, while decreased FNCs between BG and DMN in State 1. Other abnormal FNCs in TD-PDs in BG, lFPN, rFPN and vSMN were also found in State 1 and 4 compared to HCs. We also found abnormal FNCs in BG, dSMN, vSMN, and lFPN in State 1,2,3and4 in NTD-PDs ,relative to HCs.In a further analysis of correlations, we found that dwell time in State I was positively correlated with resting tremor scors in TD-PDs, possibly indicating an association between more severe tremor symptoms and prolonged length of stay in State I. In addition,dFNC strength between BG and vSMN in State3 had significant positive correlation with MDS-UPDRS-III score in PD patients ,suggesting the higher dFNC strength between BG and vSMN in State 3,the patients would have worsening motor function.Disussion
Our results show that changes in dFNC strength between BG and vSMN in the segregated State is associated with presence of tremor in Parkinson’s disease. Moreover, increased dwell time in the strongly interconnected state is related to severity of tremor symptoms in Parkinson’s disease.Our study indicates that tremor in Parkinson’s disease is characterized by altered dFNC strength and temporal properties in dynamic connectivity, which provides a new insight into the pathological of Parkinsonian tremor,and may, therefore,be used as biomarkers for the diagnosis of disorders, as well as clinical assessment. Further studies on dynamic functional connectivity changes could help to better understand the progressive dysfunction of networks between Parkinson’s disease tremor.Acknowledgements
We are grateful to all of the study participants and their familiesfor their cooperation.References
1. Kim J, Criaud M, Cho SS, Díez-Cirarda M, Mihaescu A, Coakeley S, Ghadery C, Valli M, Jacobs MF, Houle S, Strafella AP. Abnormal intrinsic brain functional network dynamics in Parkinson's disease. Brain. 2017 Nov 1;140(11):2955-2967. doi: 10.1093/brain/awx233. PMID: 29053835; PMCID: PMC5841202.
2. Li C, Fronczek-Poncelet J, Lange D, Hennecke E, Kroll T, Matusch A, Aeschbach D, Bauer A, Elmenhorst EM, Elmenhorst D. Impact of acute sleep deprivation on dynamic functional connectivity states. Hum Brain Mapp. 2020 Mar;41(4):994-1005. doi: 10.1002/hbm.24855. Epub 2019 Nov 4. PMID: 31680379; PMCID: PMC7268022.
3. Li C, Fronczek-Poncelet J, Lange D, Hennecke E, Kroll T, Matusch A, Aeschbach D, Bauer A, Elmenhorst EM, Elmenhorst D. Impact of acute sleep deprivation on dynamic functional connectivity states. Hum Brain Mapp. 2020 Mar;41(4):994-1005. doi: 10.1002/hbm.24855. Epub 2019 Nov 4. PMID: 31680379; PMCID: PMC7268022.
4. Wang J, Wang Y, Huang H, Jia Y, Zheng S, Zhong S, Chen G, Huang L, Huang R. Abnormal dynamic functional network connectivity in unmedicated bipolar and major depressive disorders based on the triple-network model. Psychol Med. 2020 Feb;50(3):465-474. doi: 10.1017/S003329171900028X. Epub 2019 Mar 14. PMID: 30868989.
5. Zhu H, Huang J, Deng L, He N, Cheng L, Shu P, Yan F, Tong S, Sun J, Ling H. Abnormal Dynamic Functional Connectivity Associated With Subcortical Networks in Parkinson's Disease: A Temporal Variability Perspective. Front Neurosci. 2019 Feb 19;13:80. doi: 10.3389/fnins.2019.00080. PMID: 30837825; PMCID: PMC6389716.
6. Ma X, Wu X, Shi Y. Changes of Dynamic Functional Connectivity Associated With Maturity in Late Preterm Infants. Front Pediatr. 2020 Jul 23;8:412. doi: 10.3389/fped.2020.00412. PMID: 32793532; PMCID: PMC7390889.
7. Schumacher J, Peraza LR, Firbank M, Thomas AJ, Kaiser M, Gallagher P, O'Brien JT, Blamire AM, Taylor JP. Dysfunctional brain dynamics and their origin in Lewy body dementia. Brain. 2019 Jun 1;142(6):1767-1782. doi: 10.1093/brain/awz069. PMID: 30938426; PMCID: PMC6536851.
8. Christiaen E, Goossens MG, Descamps B, Larsen LE, Boon P, Raedt R, Vanhove C. Dynamic functional connectivity and graph theory metrics in a rat model of temporal lobe epilepsy reveal a preference for brain states with a lower functional connectivity, segregation and integration. Neurobiol Dis. 2020 Jun;139:104808. doi: 10.1016/j.nbd.2020.104808. Epub 2020 Feb 19. PMID: 32087287.
Figures