3041
Brain structure network mediating the association between iron deposition and progression of Parkinson Disease1Department of Information and Electronics, Harbin Institute of Technology at Shenzhen, GuangDong Province, China, 2Peng Cheng laboratory, Guangdong Province, China, 3National Clinical Research Center for Geriatric Disorders, Beijing, China
Synopsis
Our study integrated a high angular resolution diffusion imaging (HARDI) and Quantitative susceptibility mapping (QSM) to clarify whether the disease-specific patterns of the associated between iron deposits and the progress of PD are affected by the structural network. In brain sturctural network of PD, we observed that the increased small-worldness and decreased rich-club coefficients significantly mediate the association between the mean QSM value in left putamen and H&Y scale by using the causal mediation analysis.
Introduction
Abnormal iron deposition has been proven to exist in the substantia nigra (SN) and striatum of Parkinson’s patients and is related to Parkinson disease (PD) progression1. Besides, many studies have reported brain network of PD were perturbed, notably decreased connections in cortical-basal ganglia motor circuit and default mode network (DMN)2. However, whether the association between iron deposition and clinical phenotype in PD is mediated by brain structure connectome remains to be investigated. To address this question, we use multimodal MRI imaging to examine the mediation effect of the brain white matter structural network on the association between iron deposition and disease progression in PD.Methods
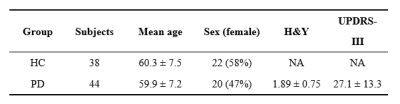
Images from 44 PD patients and 43 age-matched healthy controls (HC) recruited by Xuanwu Hospital of Capital Medical University were selected for this study. Demographic and clinical data obtained for patients included Hoehn and Yahr stage(H&Y) and motor disability (UPDRS III) scale were summarized in Table 1. All the subjects underwent Siemens Skyra 3T MR scans with an MPRAGE sequence (TR = 7 ms, TE = 3 ms, FOV = 256 × 256, thickness/gap =1.2/1.2 mm, flip angle = 8) , 3‐D single‐echo gradient echo sequence(TR = 25 ms, TE = 17.5 ms, FOV = 256 × 256, flip angle = 15)and spin echo EPI sequence (TR = 5000 ms, TE = 105 ms, FOV = 256 × 256, flip angle = 90, isotropic voxel size = 2 mm, 60 gradient directions with b = 1000/2000 s/mm2).Quantitative susceptibility mapping (QSM) reconstruction was performed using Susceptibility Tensor Imaging Suite software (STI Suite; https://people.eecs.berkeley.edu/~chunlei.liu/software.html) and using the method described in Lai Zy et al. (2019)3. We selected 10 gray‐matter brain areas as regions of interest (ROIs) manually plotted by doctors, including the SN, globus pallidus, red nucleus, caudate nucleus, and putamen. The preprocessing of DTI and Structural connectome were generated using MRtrix3 software (Tournier et al., 2019;http://mrtrix.org). The anatomical automatic labeling 2 (AAL2) atlas with 120 regions of interest was used to generate network nodes.
Network topology was computed for structure connectome by using the GRETNA toolbox (http://www.nitrc.org/projects/gretna/). We applied a sparsity threshold (0.05-0.5, with an interval of 0.05) to all brain structure network. For each sparsity level, we calculated network metrics including global efficiency, local efficiency and small-world parameters, and then calculated the area under the curve for them as a summarized scalar. The rich-club coefficients were calculated at a range of degree k value. We used R package “mediation” (http://CRAN.R-project.org/package=mediation) to test for a possible mediating role of SC topology in the association between iron deposits and H&Y stage. Disease duration was implemented in our causal mediation analysis as it has a high correlation to H&Y. The results were estimated by using the quasi-Bayesian Monte Carlo method based on normal approximation (1000 simulations).
Results
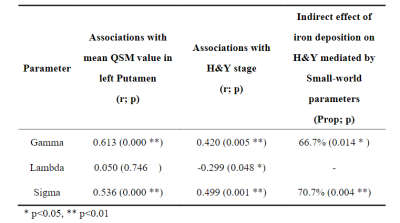
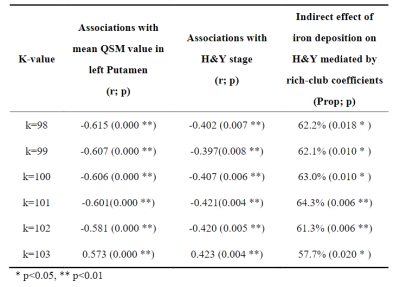
The normalized clustering coefficient “Gamma” and small-worldness sigma were positively correlated with H&Y stage and mean QSM value in left putamen in PD, while normalized characteristic path length “Lambda” only negatively correlated with H&Y stage. And the effect between mean QSM value in left putamen and H&Y stage was mediated by the coefficient Gamma and Sigma, as shown in Table 2.The rich-club coefficients were negatively correlated with mean QSM value in the putamen and H&Y stage, and rich-club coefficients significantly mediated the association of QSM value and H&Y in PD. Table 3 lists the specific values when k =98-103. However, we did not observer other significant mediation of global attributes in SC.
Discussion
Previous studies have found microstructural compensation is a normal performance in Parkinson’s disease4. In our results, increased normalized clustering coefficient and small-worldness of the brain structural network were found in PD patients, indicating abnormally increased local segregation. More importantly, the small-worldness of the brain structural network was observed to significantly mediate the association between the mean QSM value in left putamen and H&Y scale. This finding illustrates that the abnormal metabolism caused by iron deposits in PD patients may trigger compensatory mechanism to protect against a certain degree of damage.“Rich-club” is a network property that describes how high-degree network nodes are more interconnected than would be expected by chance5. In our results, the relationship between rich-club coefficients and the progressive changes in PD, suggesting the more prominent impairments of hub connectivity may depend on disease pathology. More importantly, the rich-club coefficients of the brain structural network in PD were observed to significantly mediate the association between the mean QSM value in left putamen and H&Y scale. This finding confirms that, although iron deposits mostly occur in SN and basal ganglia, they disturb structural network integrity, especially in central connections, thereby disrupting efficient communication in the brain network. A cascade of dysfunction seems to spread from the basal ganglia to the whole brain with the progression of PD.
Our findings provide a disruption pattern from iron deposits to symptoms of PD, which extend the scientific understanding of PD pathogenesis.
Acknowledgements
Acknowledgment This study is supported by grants from the Basic Research Foundation Key Project Track of Shenzhen Science and Technology Program (JCYJ20160509162237418, JCYJ20170413110656460).References
1. Guan X, Guo T, Zhou C, et al. Asymmetrical nigral iron accumulation in Parkinson's disease with motor asymmetry: an explorative, longitudinal and test-retest study. Aging-Us. Sep 30 2020;12(18):18622-18634.
2. Suo XL, Lei D, Li NN, et al. Functional Brain Connectome and Its Relation to Hoehn and Yahr Stage in Parkinson Disease. Radiology. Dec 2017;285(3):904-913.
3. Sun J, Lai Z, Ma J, et al. Quantitative Evaluation of Iron Content in Idiopathic Rapid Eye Movement Sleep Behavior Disorder. Movement Disorders. 2019;35(3):478-485.
4. Moghaddam HS, Dolatshahi M, Mohebi F, Aarabi MH. Structural white matter alterations as compensatory mechanisms in Parkinson's disease: A systematic review of diffusion tensor imaging studies. J. Neurosci. Res. Jul 2020;98(7):1398-1416.
5. Collin G, de Nijs J, Pol HEH, Cahn W, van den Heuvel MP. Connectome organization is related to longitudinal changes in general functioning, symptoms and IQ in chronic schizophrenia. Schizophrenia Research. Jun 2016;173(3):166-173.