3040
Patient-specific hyperdirect pathway activation in DBS for Parkinson’s Disease1Department of Neurosurgery, Inselspital, University Hospital Bern, Bern, Switzerland, 2ARTORG Center for Biomedical Engineering Research, University of Bern, Bern, Switzerland
Synopsis
Deep brain stimulation in the subthalamic nucleus is an effective treatment in advanced Parkinson’s disease. The aim of the current study was to determine an activation threshold of the hyperdirect pathway that results in the control of motor symptoms while avoiding the appearance of side effects. Patient-specific whole brain tractograpy, DBS leads reconstruction, and generation of volumes of tissue activated were performed in 9 subjects. The effect threshold was at 20% of hyperdirect pathway activation, while the side effect threshold was at 2% of corticospinal tract activation.
INTRODUCTION
Deep brain stimulation (DBS) is an effective treatment in advanced Parkinson’s disease patients to control motor symptoms such as bradykinesia, rigidity, or tremor. The intervention involves the precise implantation of leads to deliver pulsed electrical stimulation to a target in the basal ganglia such as the subthalamic nucleus. This nucleus plays a relevant role in the control of the motor function and is connected to the cortex through the direct, indirect and hyperdirect pathways. Recent studies suggest that stimulation of the hyperdirect pathway plays an important role in the effects of the treatment1. Clinical outcomes of DBS in Parkinson patients can be variable, and between 40 to 50% of patients report no improvement in quality of life due to persistent side effects2–4. Segmented leads can be used to reduce stimulation-induced side effects by specifically targeting regions of interest while avoiding undesired ones, improving the clinical outcome5–8. The main aim of the current study was to determine an activation threshold of the hyperdirect pathway that results in the control of motor symptoms while avoiding the appearance of side effects.METHODS
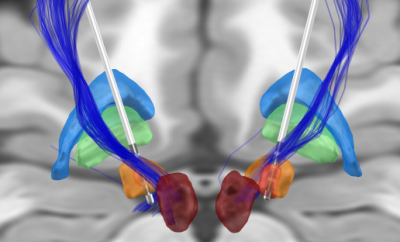
The retrospective study included 9 PD patients undergoing bilateral DBS of the STN with directional leads, as previously described by our group9. The preoperative imaging protocol included structural T1 and T2 scans and a diffusion MRI sequence (b-values in the range of 0-3000 s/mm2, 123 directions). Diffusion MRI pre-processing included denoising and unringing steps performed in MRtrix3 (v3.0), and correction of geometric distortion and eddy current artefacts in FSL (v6.0.3). As no reversed-phase encoding diffusion data were acquired, the toolbox Synb010 was used to generate an undistorted b0 image that served as an input for FSL eddy. A whole-brain tractogram of 10 million streamlines was generated in MRtrix3 (v3.0) with the probabilistic streamline algorithm iFOD211. The anatomically-constrained tractography framework was incorporated to improve diffusion MRI streamlines tractography through the use of anatomical information; and dynamic seeding was used to place seed points dynamically using the SIFT model12. The hyperdirect pathway streamlines were extracted from the whole-brain tractogram using regions of interest (ROIs) and regions of avoidance (ROAs). Brodmann Areas (BA) 4 and 6 were obtained from the Brodmann Areas Maps in FreeSurfer (v7.1). The basal ganglia structures were co-registered to patient space from the DISTAL atlas13; the substantia nigra was co-registered from the Humant Motor Thalamus Atlas14; and the striatum was co-registered from the Oxford-GSK-Imanova Structural Striatal Atlas15. To select the hyperdirect pathway streamlines, the subthalamic nucleus, and BA4 and BA6 were used as a ROIs; while the globus pallidus externa and interna, the red nucleus, the substantia nigra and the striatum were used as ROAs. Additionally, a maximum length of 90 mm was used. For obtaining the corticospinal tract, the following ROIs were used: BA4 and BA6; superior corona radiata, posterior limb of internal capsule and cerebral peduncle (JHU white-matter tractography atlas16); and medulla (Freesurfer Brainstem substructures17). The corpus callosum (JHU white-matter tractography atlas16) and the cerebellum (Freesurfer Desikan parcellation) were used as ROAs. Besides, a minimum length of 80 mm and a maximum length of 135 mm were used. DBS patients were clinically assessed six months after the implantation with a monopolar review of omnidirectional and directional DBS (e.g. effects of rigidity, tremor and bradykinesia) as previously reported by our group18. DBS leads were reconstructed using the Lead-DBS toolbox (v2.2) in Matlab 2019b, and the volume of tissue activated for each of the contacts was estimated using clinical mapping settings at effect and no effect thresholds. Finally, we calculated the percentage of pathway activation by each of the volumes of tissue activated. The activation of the hyperdirect pathway was calculated for effect thresholds, while the activation of the corticospinal tract was obtained for side effect thresholds. We trained a logistic regression model in Matlab 2019b to obtain an activation threshold between the effect and no effect, for both the hyperdirect pathway and the corticospinal tract.RESULTS
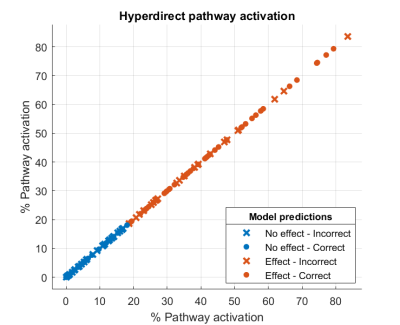
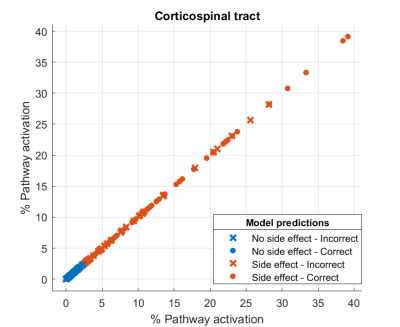
Figure 2 shows the percentages of hyperdirect pathway activation for effect and no effect. A threshold around 20% of pathway activation can established for the appearance of the effect. The accuracy of the logistic regression model was 61,5%. Figure 3 shows the percentages of corticospinal tract activation for side effect and no side effects. A threshold around 2% of pathway activation can established for the appearance of side effects. The accuracy of the logistic regression model was 63%.DISCUSSION
Although an activation threshold for the appearance of effect and side effects was established, the accuracy of the trained logistic regression models was quite low. Training the model with more data points could possibly increase the accuracy. On the other side, inferring pathway activation according to the percentage of activated streamlines might not be the ideal solution. The filtering algorithm SIFT212 allows inferring connection density as the sum of streamlines weights to obtain the fiber bundle connectivity. This metric might be more appropriate when obtaining activation thresholds across subjects.CONCLUSION
Obtaining a more accurate activation threshold of the hyperdirect pathway would allow in the future more informed and precise targeting of the hyperdirect pathway in DBS treatments for Parkinson’s disease.Acknowledgements
No acknowledgement found.References
1. Gradinaru, V., Mogri, M., Thompson, K. R., Henderson, J. M. & Deisseroth, K. Optical deconstruction of parkinsonian neural circuitry. Science (80-. ). 324, 354–359 (2009).
2. Dafsari, H. S. et al. Short-term quality of life after subthalamic stimulation depends on non-motor symptoms in Parkinson’s disease. Brain Stimul. 11, 867–874 (2018).
3. Floden, D., Cooper, S. E., Griffith, S. D. & Machado, A. G. Predicting quality of life outcomes after subthalamic nucleus deep brain stimulation. Neurology 83, 1627–1633 (2014).
4. Daniels, C. et al. Is improvement in the quality of life after subthalamic nucleus stimulation in Parkinson’s disease predictable? Mov. Disord. 26, 2516–2521 (2011).
5. Pollo, C. et al. Directional deep brain stimulation: an intraoperative double-blind pilot study. Brain 137, 2015–2026 (2014).
6. Steigerwald, F., Müller, L., Johannes, S., Matthies, C. & Volkmann, J. Directional deep brain stimulation of the subthalamic nucleus: A pilot study using a novel neurostimulation device. Mov. Disord. 31, 1240–1243 (2016).
7. Contarino, M. F. et al. Directional steering: A novel approach to deep brain stimulation. Neurology 83, 1163–1169 (2014).
8. Dembek, T. A. et al. Directional DBS increases side-effect thresholds—A prospective, double-blind trial. Mov. Disord. 32, 1380–1388 (2017).
9. Nowacki, A. et al. Accuracy of different three-dimensional subcortical human brain atlases for DBS –lead localisation. NeuroImage Clin. 20, 868–874 (2018).
10. Schilling, K. G. et al. Distortion correction of diffusion weighted MRI without reverse phase-encoding scans or field-maps. PLoS One 15, e0236418 (2020).
11. Tournier, J. D., Calamante, F. & Connelly, A. MRtrix: Diffusion tractography in crossing fiber regions. Int. J. Imaging Syst. Technol. 22, 53–66 (2012).
12. Smith, R. E., Tournier, J. D., Calamante, F. & Connelly, A. Anatomically-constrained tractography: Improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage 62, 1924–1938 (2012).
13. Ewert, S. et al. Toward defining deep brain stimulation targets in MNI space: A subcortical atlas based on multimodal MRI, histology and structural connectivity. NeuroImage 170, 271–282 (2018).
14. Ilinsky, I. et al. Human motor thalamus reconstructed in 3D from continuous sagittal sections with identified subcortical afferent territories. eNeuro 5, 60–78 (2018).
15. Tziortzi, A. C. et al. Imaging dopamine receptors in humans with [11C]-(+)-PHNO: Dissection of D3 signal and anatomy. Neuroimage 54, 264–277 (2011).
16. Hua, K. et al. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage 39, 336–347 (2008).
17. Iglesias, J. E. et al. Bayesian segmentation of brainstem structures in MRI.
18. Nguyen, T. A. K. et al. Analysis of patient-specific stimulation with segmented leads in the subthalamic nucleus. PLoS One 14, e0217985 (2019).
Figures