3038
Brain white and gray matter alterations in early-stage Parkinson's disease with GBA1 gene mutations evaluated using free water imaging1Department of Radiology, Juntendo University Graduate School of Medicine, Tokyo, Japan, 2Department of Radiological Sciences, Graduate School of Human Health Sciences, Tokyo Metropolitan University, Tokyo, Japan, 3Department of Radiology, Toho University Omori Medical Center, Tokyo, Japan
Synopsis
Neuropathological mechanisms of Parkinson’s disease with GBA1 mutations (GBA-PD) are still unclear. We evaluated the white and gray matter of patients with early-stage GBA-PD using free water (FW) imaging. Patients with GBA-PD showed higher FW-corrected fractional anisotropy and lower FW-corrected mean diffusivity in the anterior thalamic radiation, forceps minor, and uncinate fasciculus, reflecting compensatory reorganization of neural circuits. Meanwhile, neuroinflammation (indexed by higher FW) was detected in the substantia nigra pars compacta (SNpc; Braak stage III), temporal mesocortex (Braak stage IV), forceps major, and cingulum cingulate gyrus. Furthermore, FW in the SNpc was positively correlated with the motor assessment scale.
INTRODUCTION
Parkinson’s disease (PD) is characterized by a broad spectrum of motor and non-motor symptoms.1 Most PD cases are idiopathic, with 10–15% being associated with gene mutations. Mutations in GBA1, the gene encoding lysosomal enzyme glucocerebrosidase (GCase), are among the most common known genetic risk factors for PD development.2 Motor progression tends to be more aggressive in PD with GBA1 mutations (GBA-PD) with a higher incidence of non-motor features compared to idiopathic PD (iPD).2 The neuropathological mechanisms of GBA-PD are still unclear. However, recent studies highlighted the association between GCase defects and neuroinflammation in the brain parenchyma.2 In the current study, we applied free water (FW) imaging to investigate differences in white matter (WM) and gray matter (GM) integrity between iPD and GBA-PD. FW-corrected diffusion tensor imaging parameters for partial volume effects from extracellular FW using a bi-tensor model allow better description of tissue microstructure.3 The model also estimates the fractional volume of FW in a voxel that can be used to monitor changes in the extracellular space, such as neuroinflammation.4METHODS
SubjectsWe analyzed the clinical and MRI data of patients with early-stage drug-naïve PD (GBA-PD, N = 19; iPD, N = 104) and age- and sex-matched healthy controls (HC, N = 34; Table 1) from the Parkinson’s Progression Markers Initiative database (http://www.ppmi-info.org/).5, 6
MRI acquisition and processing
The 3D T1-WI and DWI were performed using a standardized protocol on 3-T Siemens scanners. An in-house MATLAB script was used to fit a regularized bi-tensor model and generate FW-corrected fractional anisotropy (FAT), FW-corrected mean diffusivity (MDT), and FW maps.
WM probabilistic tractography
Major WM pathways (forceps major [Fmajor], forceps minor [Fminor], anterior thalamic radiation [ATR], cingulum angular bundle, cingulum cingulate gyrus [CCG], corticospinal tract, inferior longitudinal fasciculus [ILF], superior longitudinal fasciculus parietal and temporal, and uncinate fasciculus [UF]) were obtained using TRActs Constrained by UnderLying Anatomy (TRACULA; Figure 1).7
GM region-of-interest (ROI) analysis
GM areas were parcellated using automatic anatomical labeling Atlas 38 and Desikan-Killiany atlases.9 GM areas were evaluated based on the Braak staging, which comprised substantia nigra pars compacta (SNpc; stage III); temporal mesocortex (stage IV); frontal, temporal, and parietal association cortex (stage V); and motor and sensory neocortex (stage VI).10
Cortical volume and thickness measurements
Cortical volume and thickness in all subjects were obtained using FreeSurfer (Fs) version 6.0.0. with the recon-all pipeline.11
Statistical analysis
Mean FAT, MDT, and FW values in the WM and GM were compared between groups using one-way analysis of covariance, and Bonferroni-corrected P-value < 0.05 was considered statistically significant. Meanwhile, Fs-Permutation Analysis of Linear Models (voxel-wise cluster forming threshold = 1.3, 1000 permutation iterations, and cluster-wise P < 0.05) were used to compare the cortical volume and thickness between groups. Age, sex, and scanning site were included as nuisance covariates in group comparison analysis. The clinical scores were also correlated with FAT, MDT, and FW in the WM and GM using Pearson’s correlation analysis.
RESULTS
WM probabilistic tractography (Figure 2): Significantly higher FAT was demonstrated in Fminor and UF in GBA-PD compared to HC and iPD and ATR in GBA-PD compared to HC. Moreover, significantly lower MDT was found in Fminor and ATR of GBA-PD compared to HC and iPD. GBA-PD had significantly higher FW in Fmajor and CCG compared to iPD and in ILF compared to HC.GM ROI analysis (Figure 3): Significantly higher FW was detected in SNpc (Braak stage III) in GBA-PD compared to HC and iPD and in the temporal mesocortex (Braak stage IV) in GBA-PD compared to HC.
Cortical volume and thickness measurements: No significant findings were demonstrated between groups.
Correlation analysis (Figure 4): A significant positive correlation was demonstrated between FW and Movement Disorder Society’s Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS III) score in SNpc in GBA-PD.
DISCUSSION
The current study investigated WM and GM alterations in PD with GBA1 mutations using FW imaging. Increased WM integrity (indexed by higher FAT and lower MDT) in some major WM pathways (ATR, Fminor, and UF) in GBA-PD may reflect the compensatory reorganization of neural circuits.12 The reduction in GCase activity leads to an increase in α-synuclein accumulation and aggregation in Lewy bodies and neurites, the pathological hallmark of PD.2, 13 Recently, the accumulation of α-synuclein, resulting in axonal arborization, has been demonstrated in early-stage α-synuclein transgenic mouse brain.14 Meanwhile, neuroinflammation (indexed by higher FW)15 was detected in the SNpc (Braak stage III), temporal mesocortex (Braak stage IV), Fmajor, and CCG in GBA-PD. Low GCase activity has also been associated with activation of astrocytosis and microglia in the brain parenchyma. A post-mortem analysis of human GBA-PD brains exhibited extensive neuroinflammation in the substantia nigra.5 Furthermore, the neuroinflammation biomarker might also be used for evaluating motor symptom progression, reinforced by the positive correlation between FW and MDS-UPDRS III in the SNpc in GBA-PD.CONCLUSION
FW imaging increase the understanding of WM and GM pathologies in patients with early-stage drug-naïve GBA-PD and might be used for disease progression biomarkers. Based on the results, we suggest the role of GBA1 mutations in brain compensatory mechanisms and neuroinflammation, which provides opportunities for developing disease-modifying therapies and disease progression biomarkers.Acknowledgements
The funding sources of Parkinson’s Progression Markers Initiative is available on www.ppmi-info.org/fundingpartners. CA is an overseas researcher under Postdoctoral Fellowship of Japan Society for the Promotion of Science (JSPS).References
- Jankovic J. Parkinson's disease: Clinical features and diagnosis. J Neurol Neurosurg Psychiatry 2008;79:368-76.
- Avenali M, Blandini F, Cerri S. Glucocerebrosidase defects as a major risk factor for Parkinson's Disease. Front Aging Neurosci 2020;12:97.
- Pasternak O, Westin CF, Bouix S, et al. Excessive extracellular volume reveals a neurodegenerative pattern in schizophrenia onset. J Neurosci 2012;32:17365-72.
- Pasternak O, Sochen N, Gur Y, et al. Free water elimination and mapping from diffusion MRI. Magn Reson Med 2009;62:717-30.
- Marek K, Chowdhury S, Siderowf A, et al. The Parkinson's progression markers initiative (PPMI) - establishing a PD biomarker cohort. Ann Clin Transl Neurol 2018;5:1460-77.
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A 2000;97:11050-5.
- Yendiki A, Panneck P, Srinivasan P, et al. Automated probabilistic reconstruction of white-matter pathways in health and disease using an atlas of the underlying anatomy. Front Neuroinform 2011;5:23.
- Rolls ET, Huang CC, Lin CP, et al. Automated anatomical labelling atlas 3. Neuroimage 2020;206:116189.
- Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 2006;31:968-80.
- Braak H, Del Tredici K. Invited article: Nervous system pathology in sporadic Parkinson disease. Neurology 2008;70:1916-25.
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 1999;9:179-94.
- Mole JP, Subramanian L, Bracht T, et al. Increased fractional anisotropy in the motor tracts of Parkinson's disease suggests compensatory neuroplasticity or selective neurodegeneration. Eur Radiol 2016;26:3327-35.
- Schapira AH. Glucocerebrosidase and Parkinson disease: Recent advances. Mol Cell Neurosci 2015;66:37-42.
- Schechter M, Grigoletto J, Abd-Elhadi S, et al. A role for alpha-Synuclein in axon growth and its implications in corticostriatal glutamatergic plasticity in Parkinson's disease. Mol Neurodegener 2020;15:24.
- Wang Y, Wang Q, Haldar JP, et al. Quantification of increased cellularity during inflammatory demyelination. Brain 2011;134:3590-601.
Figures
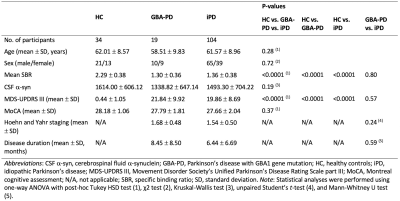
Table 1. Demographic characteristics of study participants
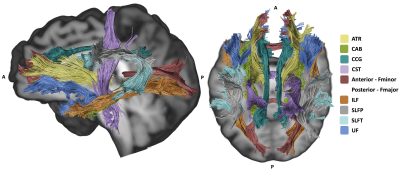
Figure 1. Major white matter pathways reconstructed with TRActs Constrained by UnderLying Anatomy (TRACULA).
Abbreviations: ATR, anterior thalamic radiation; CAB, cingulum angular bundle; CCG, cingulum cingulate gyrus; CST, corticospinal tract; Fmajor, forceps major; Fminor, forceps minor; ILF, inferior longitudinal fasciculus; SLFP, superior longitudinal fasciculus parietal; SLFT, superior longitudinal fasciculus temporal; and UF, and uncinate fasciculus.
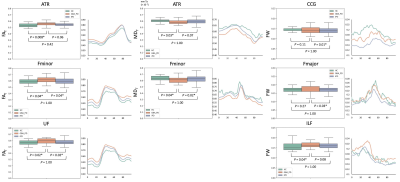
Figure 2. Box plots and tract profiles of FAT, MDT, and FW for HC, GBA-PD, and iPD groups in the white matter pathways.
Abbreviations: ATR, anterior thalamic radiation; CCG, cingulum cingulate gyrus; FAT, free water-corrected fractional anisotropy; Fmajor, forceps major; Fminor, forceps minor; FW, free water; GBA-PD, Parkinson’s disease with GBA1 mutations; HC, healthy controls; ILF, inferior longitudinal fasciculus; iPD, idiopathic Parkinson’s disease; MDT, free water-corrected mean diffusivity; UF, uncinate fasciculus.
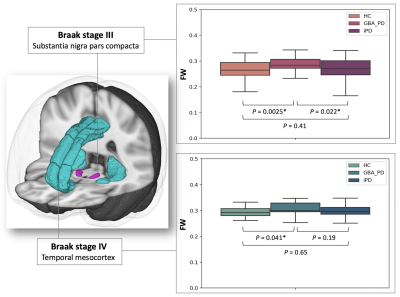
Figure 3. Box plots of FW for the HC, GBA-PD, and iPD groups in Braak stages III and IV. The bottom and top of the box are the first and third quartiles, and the thick band inside the box is the median. Whiskers represent maximum and minimum values of all data.
Abbreviations: FW, free water, GBA-PD, Parkinson’s disease with GBA1 mutations; HC, healthy controls; and iPD, idiopathic Parkinson’s disease.
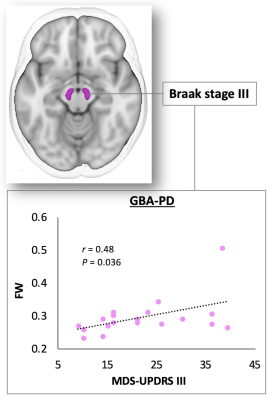
Figure 4. Scatterplots showing a positive correlation in the Braak stage III between FW and MDS-UPDRS III score in patients with GBA-PD.
Abbreviations: FW, free water; GBA-PD, Parkinson’s disease with GBA1 mutations; MDS-UPDRS III, Movement Disorder Society’s Unified Parkinson’s Disease Rating Scale part III.