3037
Excessive Brain Iron Deposition Detected by QSM in Extrapyramidal System in Parkinson's with Type 2 Diabetes Melitus Patients1First Affiliated Hospital of Dalian Medical University, Dalian, China
Synopsis
Type 2 diabetes melitus(T2DM) is associated with worse motor imbalance and cognitive decline in Parkinson’s disease.Abnormal cerebral iron deposition occur in extrapyramidal system in PD patients. However, whether the microscopic iron deposition will increase further in Parkinson’s patients with T2DM remains unknown. In this study, the iron deposition in extrapyramidal system measured QSM was compared between Parkinson's patients with T2DM (PDDM) and Parkinson's patients without T2DM (PDND).The increased magnetic sensitivity values (MSV) were found in most brain gray matter nucleus,especially for the left globus pallidus(GP) and bilateral substantia nigra(SN),in PDDM group rather than in PDND group.
Introduction
The large epidememiology studies point out that PD patients with diabetes show worse motor symptoms, such as postural instability and gait difficulties, compared to non-diabetic cases[1].For PD patients, excess iron deposition in extrapyramidal,has a clear relationship with clinical dysfunction.The excessive cerebral iron deposition also exists in T2DM.However whether PDDM further aggravates iron deposition is not clear.Quantitative Susceptibility Mapping (QSM) has been shown to be a promising modality for the diagnosis of PD which provides both the quantitative information of the regional iron content and also its distribution within gray nuclei[2].As a result,we aim to explore the the MSV difference between the PDDM and the PDND group,and further to analyze the relation of iron deposition with clinical data.Materials and Methods
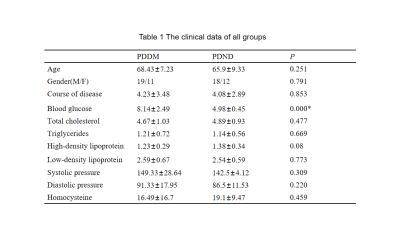
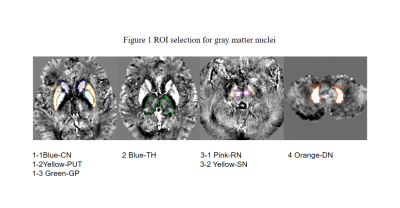
This retrospective study was approved by the hospital ethics committee. Thirty PDDM patients (mean age:68.43±7.23 years,range:58-81 years,11 female) and thirty PDND patients(mean age:65.9±9.33 years,range:47-85 years,12 female) were recruited with informed consent acquired from each subject.The clinical data are collected as shown in Table 1.All patients performed conventional MRI protocols and gradient echo T2 star weighted angiography (ESWAN) using GE signa HDXT 3.0T MRI scanner with8-channel head coil.The ESWAN parameters were kept the same (TR=36ms,TE=3.6ms;7.8ms;11.9ms;16.1ms;20.3ms;24.4ms;28.6ms;32.8ms,FOV =24x24cm2,slice thickness=1mm,slice gap=0mm,matrix=256x256).The QSM images were post-processed by using signal processing in nuclear magnetic resonance (SPIN) software. The region of interest (ROI) is set manually along the edge of bilateral caudate nucleus (CN), putamen (PUT), globus pallidus (GP), thalamus (TH), substantia nigra (SN), red nucleus (RN), and dentate nucleus (DN), as shown in Figure 1. The MSV in every nucleus was measured three times.
The mean MSV and clinical data (age, total cholesterol, triglyceride, high-density lipoprotein, low-density lipoprotein, homocysteine) was compared between groups by independent sample t test.The correlation between clinical data and MSV in the PDDM group were performed by Pearson correlation analysis(P<0.05 is significant differences).
Results
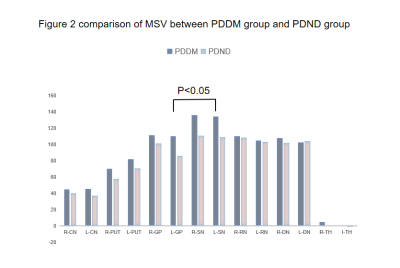
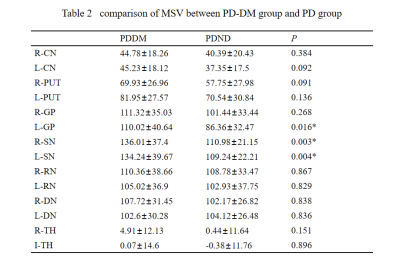
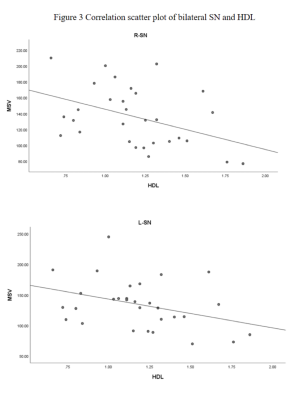
From Table 2,except for the left dentate nucleus, the MSVs of the PDDM group were slight greater than those of the PDND group, and there are significant differences in bilateral SN and left GP(P<0.05),as shown in Figure 2.And there is no significant difference between the other groups of gray matter nucleus.The MSV of left SN(r =-0.364, P =0.048) and right SN(r=-0.415,P=0.023) negatively correlated with high-density lipoprotein (HDL) content,as shown in Figure 3.Discussion
This study reports the effect of type 2 diabetes on the MSV value of the extrapyramidal system in Parkinson's patients. and the significant difference exists in the left GP and bilateral SN.Experimental studies support the hypothesis that insulin resistance exists in patients with type 2 diabetes.It reduces survival signals and enhances mitochondrial dysfunction and oxidative stress and local inflammation, which ultimately culminates in activation of an apoptotic pathway of PD-related nigrostriatal neurodegeneration,and consequently leads to a striatal dopamine deficit. Previous studies have found that the activity of peroxisome proliferator-activated receptor γ-coactivator 1-α (PGC1α) is reduced in both diseases,and PGC1α is a transcriptional regulator that mediates mitochondrial biogenesisal[3].Though the exact mechanism is not clear, DM and PD may partly share some common pathophysiology pathways[4].Compared with non-diabetic patients, Parkinson's disease with diabetes patients show more severe symptoms.The brain iron content of the substantia nigra and globus pallidus is significantly linearly correlated with the severity of the disease.Conclusion
Our results display that diabetes can aggravate iron deposition due to the abnormal metabolism of dopamine in PD patients.Acknowledgements
Thanks for the guidance of graduate tutor MiaoReferences
[1] Gentile F,Doneddu PE,Riva N,Quattrini A,et al.Diet, Microbiota and Brain Health: Unraveling the Network Intersecting Metabolism and Neurodegeneration.Int J Mol Sci.2020 Oct 10 ;21(20)
[2]Xiao B,He N,Wang Q,Shi F,et al.Quantitative susceptibility mapping based hybrid feature extraction for diagnosis of Parkinson's disease.Neuroimage Clin.2019;24:102070.
[3]Moroo I, Yamada T, Makino H, et al. Loss of insulin receptor immunoreactivity from the substantia nigra pars compacta neurons in Parkinson's disease. Acta Neuropathol 1994;87:343-348.
[4]Aviles-Olmos, I.; Limousin, P .; Lees, A.; Foltynie, T. Parkinson’s disease, insulin resistance and novel agents of neuroprotection. Brain J. Neurol. 2012, 136, 374–384. [CrossRef]