3036
Exposure to Traffic-Related Particulate Matter Impact Motor Function and White Mater Integrity in Elder Rodent Model1Biomedical Engineering, National Yang-Ming University, Taipei, Taiwan, 2Ph.D. Program for Neural Regenerative Medicine, Taipei Medical University, Taipei, Taiwan, 3School of Respiratory Therapy, College of Medicine, Taipei Medical University, Taipei, Taiwan
Synopsis
Exposure to air pollution was demonstrated to be correlated with the neurodegenerative disease showing the cognitive deficits, but the impacts on motor function has yet to be investigated. The motor behavioral test and diffusion magnetic resonance imaging (MRI) tractography analysis were applied to the traffic-related particulate matter (PM)-exposed models in this study. We found the decline in motor function and the destruction of the microstructure after exposed to traffic-related PM for 3 months, in which the structural alterations of motor control circuit were similar to the characteristic of the Parkinson's disease (PD).
Introduction
Recent evidence indicates that particulate matter (PM)-induced neurodegenerative changes could be key factors in the pathogenesis of many central nervous system disorders including Parkinson’ disease (PD)1. One of the possible routes of PM entering the brain has been reported to be via the olfactory neurons directly2. Once the PM gets into the brain, it may induce exceed microglial activation which could lead to white matter (WM) injury3. Recently, the existing evidence revealed that the PM is associated with cognitive decline and behavioral deficits4. However, the PM‐induced motor function disorders have not been elucidated. The basal ganglia circuit including the nigrostriatal dopamine tract plays an important role in motor control5. Furthermore, it has been demonstrated that PM could damage of dopaminergic neurons in the substantia nigra (SN) which is considered as the neuropathologic feature of PD6. Besides, the dopaminergic pathway was also demonstrated to be essential for modulating cognitive functions7. In this study, we aimed to identify whether there were PM‐induced motor function defects and the effects on WM integrity using diffusion tensor imaging (DTI). To address these objectives, elder Sprague-Dawley (SD) rats, as the susceptible population to PM2.5‐induced neurotoxicity8, were exposed to traffic-related PM for 3 months, followed by a brain magnetic resonance imaging (MRI) tractography analysis and behavioral observations.Methods
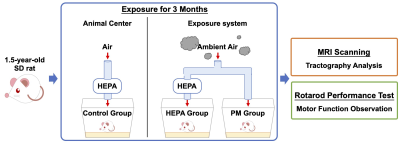
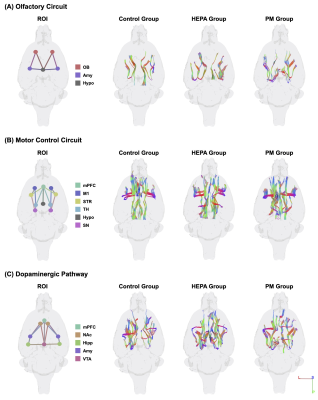
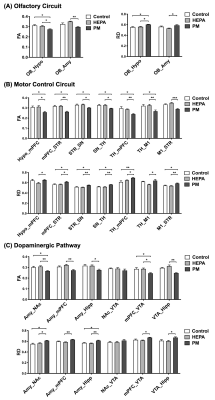
Thirty male Sprague-Dawley (SD) rats (1.5 years old) were supplied by the National Laboratory Animal Center. All rats were maintained at a constant temperature of 22 ± 2 °C with a relative humidity of 55 % ± 10 % and a 12 h light/dark cycle. To investigate the impacts of chronic pulmonary exposure to traffic-related PM, the rats were randomly divided into the following three groups: (1) a control group (N = 10), living in a clean air (housed in the Laboratory Animal Center); (2) a high-efficiency particulate air (HEPA) group (N = 10), provided with a HEPA-filtered air (exposed to gaseous pollution only); (3) a PM group (N = 10), exposed to PM and gaseous pollution. PM was collected from the downtown near a highway and expressway (New Taipei City, Taiwan), introduced to a whole-body exposure system used in a previous study9. Following three months of exposure, rats were conducted with MRI scanning and rotarod performance test to analyze brain tractography and determine motor function, respectively (Figure 1). Whole brain images were acquired from a custom-made 3 T MRI system10. DTI data were acquired in 25 directions using DTI echo planar imaging sequence with the following parameters: repetition time = 2,000 ms, echo time = 65 ms, 18 coronal slices, slice thickness = 1 mm, field of view = 40 × 40 mm2, matrix size = 128 × 128. The regions of interest (ROIs) were determined according to the rat brain atlas11, including the olfactory bulb (OB), medial prefrontal cortex (mPFC), primary motor cortex (M1), nucleus accumbens (NAc), striatum (STR), thalamus (TH), hippocampus (Hipp), amygdala (Amy), hypothalamus (Hypo), SN and ventral tegmental area (VTA) (Figure 2). Then, the tractographic analysis was performed using the DSI studio12, and the fractional anisotropy (FA) and radial diffusivity (RD) were exported to respectively indicate the fiber integrity and myelination. The rotarod performance test was considered as motor coordination and balance13, and the latency taken for the rat to stay on the rotarod was measured. For our data, significant differences among multiple groups were obtained by the Kruskal–Wallis test followed by the Dunn’s multiple comparisons test. Data were considered as statistically significant at p-value < 0.05. Data were presented as the mean ± standard error of the mean.Results
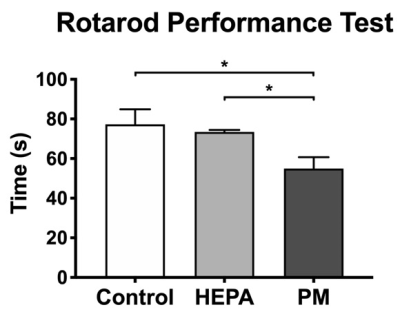
The track-specific analysis of brain regions associated with motor and memory function showed that there were significant decreased FA and increased RD values of the firer tracts in the PM group compared to control group and HEPA group (Figure 3). In the rotarod performance test, we observed that the rats in the PM group spent less time staying on the rotarod, indicating the motor function deficit after 3 months of traffic-related PM exposure (Figure 4).Discussion
Our DTI data demonstrated that chronic exposure to traffic-related PM may damage the structure of the neuron and result in decreased FA and increased RD, which indicated the impairment in fiber integrity and the degradation in myelinated fibers14, respectively. As the previous studies showed, air pollution exposure may lead to olfactory deficits with the OB pathology15. The damages of basal ganglia neurocircuit were thought to be associated with the motor deficits characteristic of PD16; besides, our results of rotarod performance test also demonstrated the changes of motor function. Additionally, the degeneration of dopaminergic neurons has been implicated in the cognitive dysfunction in PD17.Conclusion
The findings demonstrated that the chronic exposure to traffic-related PM caused WM impairments and led to motor function defects. After exposure to PM, the alterative microstructures of the olfactory circuit, motor control circuit and dopaminergic pathway could be the possible progressions for the development of neurological disorders as well as a potential risk for PD.Acknowledgements
This work is financially supported by Ministry of Science and Technology of Taiwan under Contract numbers of MOST 109-2221-E-010-004-MY2, 109-2314-B-303-016, and 108-2321-B-010-008-MY2. We also are grateful for support from the Higher Education Sprout Project of the National Chiao Tung University, Headquarters of University Advancement at the National Cheng Kung University.
References
1. Brockmeyer S, D’Angiulli A. How air pollution alters brain development: the role of neuroinflammation. Translational neuroscience. 2016;7(1):24-30.
2. Oberdörster G, Sharp Z, Atudorei V, et al. Translocation of inhaled ultrafine particles to the brain. Inhalation toxicology. 2004;16(6-7):437-445.
3. Nephew BC, Nemeth A, Hudda N, et al. Traffic-related particulate matter affects behavior, inflammation, and neural integrity in a developmental rodent model. Environmental Research. 2020;183:109242.
4. Calderón-Garcidueñas L, Mora-Tiscareño A, Ontiveros E, et al. Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain and cognition. 2008;68(2):117-127.
5. Allen JL, Liu X, Weston D, Conrad K, Oberdörster G, Cory-Slechta DA. Consequences of developmental exposure to concentrated ambient ultrafine particle air pollution combined with the adult paraquat and maneb model of the Parkinson's disease phenotype in male mice. Neurotoxicology. 2014;41:80-88.
6. Loane C, Pilinis C, Lekkas TD, Politis M. Ambient particulate matter and its potential neurological consequences. Reviews in the Neurosciences. 2013;24(3):323-335.
7. Rieckmann A, Karlsson S, Karlsson P, et al. Dopamine D1 receptor associations within and between dopaminergic pathways in younger and elderly adults: links to cognitive performance. Cerebral Cortex. 2011;21(9):2023-2032.
8. Shou Y, Huang Y, Zhu X, Liu C, Hu Y, Wang H. A review of the possible associations between ambient PM2. 5 exposures and the development of Alzheimer's disease. Ecotoxicology and environmental safety. 2019;174:344-352.
9. Yan Y-H, Chou CC-K, Wang J-S, et al. Subchronic effects of inhaled ambient particulate matter on glucose homeostasis and target organ damage in a type 1 diabetic rat model. Toxicology and applied pharmacology. 2014;281(2):211-220.
10. Cho K-H, Huang S-M, Choi C-H, et al. Development, integration and use of an ultra-high-strength gradient system on a human-size 3 T magnet for small animal MRI. PloS one. 2019;14(6):e0217916.
11. Papp EA, Leergaard TB, Calabrese E, Johnson GA, Bjaalie JG. Waxholm Space atlas of the Sprague Dawley rat brain. Neuroimage. 2014;97:374-386.
12. Yeh F-C, Verstynen TD, Wang Y, Fernández-Miranda JC, Tseng W-YI. Deterministic diffusion fiber tracking improved by quantitative anisotropy. PloS one. 2013;8(11):e80713.
13. Lepannetier S, Gualdani R, Tempesta S, et al. Activation of TRPC1 channel by metabotropic glutamate receptor mGluR5 modulates synaptic plasticity and spatial working memory. Frontiers in cellular neuroscience. 2018;12:318.
14. Mori S, Zhang J. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron. 2006;51(5):527-539.
15. Calderón-Garcidueñas L, Franco-Lira M, Henríquez-Roldán C, et al. Urban air pollution: influences on olfactory function and pathology in exposed children and young adults. Experimental and Toxicologic Pathology. 2010;62(1):91-102.
16. Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543-554.
17. Schapira AH, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nature Reviews Neuroscience. 2017;18(7):435.
Figures