3031
STN/GP-nets: Fully automatic deep-learning based segmentation for DBS applications using ultra-high 7 Tesla MRI1Center for Magnetic Resonance Research, Department of Radiology, University of Minnesota, Minneapolis, MN, United States, 2Department of Neurology, University of Minnesota, Minneapolis, MN, United States, 3Department of Neurosurgery, University of Minnesota, Minneapolis, MN, United States, 4Department of Electrical and Computer Engineering, Department of Biomedical Engineering, Department of Computer Science, Department of Mathematics, Duke University, Durham, NC, United States
Synopsis
Deep brain stimulation (DBS) surgery has been shown to improve the quality of life for patients with various motor dysfunctions. The success of DBS is directly related to the proper placement of the electrodes, which requires accurate detection and identification of the relevant target structures. We present a deep-learning based automatic, robust and accurate segmentation technique from 7 Tesla MRI acquisitions of subcortical structures for DBS surgery planning and post-operative electrode localization. DBS targets and related structures include the subthalamic nucleus, substantia nigra, red nucleus and the internal and external compartments of the globus pallidus.
Introduction
Deep brain stimulation (DBS) therapy has shown clear clinical efficacy in the mediation of symptomatic motoric behavior associated with neurological disorders such as Parkinson’s disease (PD). Recent studies have shown that accurate placement of the DBS electrode within the sensorimotor region of the target (e.g. subthalamic nucleus (STN) or globus pallidus interna (GPi)) is directly correlated with the success of the DBS procedure and reduction of adverse effects1,2. Thus, precise identification and visualization of DBS targets is of great importance. A fully automated segmentation process of DBS targets has several clear advantages, among which are accurate and fast inference, the potential to streamline clinical workflow and increase patient throughput, both in the pre-operative surgery planning and in the post-operative assessment of the DBS lead localization with respect to the target, for patient programming purposes. We present two fully convolutional deep neural networks, STN-net and GP-net; STN-net for the segmentation of the STN, substantia nigra (SN) and red nucleus (RN), and GP-net for the segmentation of the internal and external compartments of the globus pallidus (GP) from 7 Tesla (T) T2 acquisitions.Methods
Patients were scanned on a 7 T MRI scanner (Magnetom 7 T Siemens, Erlangen, Germany) using our previous published protocols 3,4. The scanner was equipped with SC72 gradients capable of 70 mT/m and a 200 T/m/s slew rate using a 32-element head array coil (Nova Medical, Inc., Burlington, MA, USA). Whenever patient head size enabled enough space in the coil, dielectric pads were utilized in order to enhance signal in the temporal regions5. The scan protocol consists of a T2-weighted axial slab covering from the top of the thalamus to the bottom of the SN with 0.39 x 0.39 x 1 mm3 resolution (for GP-net training and inference) and a coronal slab covering from the anterior commissure to the 4th ventricle with 0.39 x 1 x 0.39 mm3 resolution (for STN-net training and inference). In total, 101 patient axial scans were used to train GP-net (58 patients for training and 43 for testing) and 135 patient coronal scans were used to train STN-net (96 for training and 39 for testing). Both networks are based on the attention-gated U-net architecture6 and deformable convolutions7. Networks are trained end-to-end in a fully supervised manner. Manual 3D delineations (Ground-truth) performed by domain experts for the target structures per each patient’s 7 T T2 scan. The T2 volumes and manual segmentations were resampled to an isotropic grid of 0.39 mm3 prior to training and inference. All of the quantitative analysis is performed on the resampled grid. We compare the performance of both networks against state-of-the-art atlas-based segmentation, by registering the atlases into each patient’s T2 space.Results
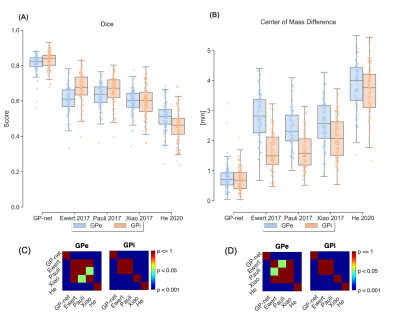
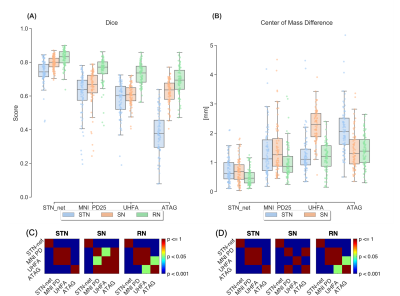
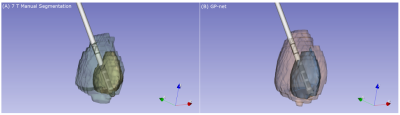
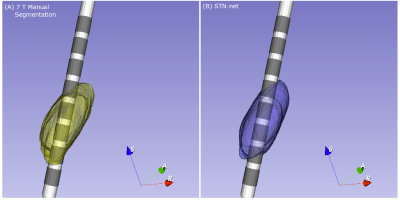
Figure 1A shows dice scores for GP-net and the selected atlas-based segmentations, both for the GPi and GPe (GP externa), individually, while Fig. 1B shows the center of mass (CoM) distance (mm) between the resulting segmentations and the manual delineations. In all of these figures, the metrics are calculated per patient, per structure, and per hemisphere. Figures 2A and 2B present the same comparison for STN-net. In both figures, panels (C) and (D) indicate the statistical significance between each segmentation method (i.e. deep network or atlas) and the other methods for panels (A) and (B), respectively. The p-value matrices show clear statistically significant differences between the networks and the other atlas-based segmentations. GP-net and STN-net both outperform all other atlas-based segmentations, achieving higher dice values (“what is the shape?”) and lower CoM errors (“where is the structure?”). Figures 3 and 4 present a post-operative assessment of DBS lead location with respect to the 3D reconstructions of the GP (GP-net) and STN (STN-net). In both figures, excellent agreement is observed both by the location and shape of the networks’ output and the manual delineations, as well as for the relative location to the leads in respect to the structures’ borders.Discussion and Conclusion
We presented two deep-learning frameworks for the segmentation of DBS related subcortical targets. Both GP-net and STN-net are able to produce accurate and reliable segmentations in a fully automated manner from 7 T T2 MR acquisitions. Both networks demonstrate better accuracy over contemporary atlas-based segmentations when compared to manual segmentations. Although the networks are described in the context of DBS surgery, all clinical procedures which require pre-surgery GP/STN trajectory planning, such as magnetic resonance guided focused ultrasound8, can benefit from this method. Contrary to atlas-based segmentations, GP-net and STN-net rely solely on 7 T T2 scans to perform inference. No registrations are involved in the process, both are segmented directly in the patient’s T2 image space and therefore, bypassing one of the deficiencies of atlas-based approaches. With the incorporation of advanced DBS pre-surgery targeting and post-surgery lead localization tools and software, 7 T MRI based approaches, either for training or for deployment, have great potential in becoming clinical standards, especially now that the 7 T MRI is FDA approved for standard clinical applications. In this scenario, the use of fully automated segmentation software may prove to be very advantageous, leading to an accurate, fast, easy and reliable visualization tool, contributing to an improved surgical procedure and patient experience.Acknowledgements
This work was supported in part by R01-NS081118, R01-NS113746, P30-NS076408, P41-EB027061 and the University of Minnesota Udall center P50NS098573.References
1. Paek, S. H. et al. The clinical impact of precise electrode positioning in STN DBS on three-year outcomes. J. Neurol. Sci. 2013; 327, 25–31.
2. Rolston, J. D., Englot, D. J., Starr, P. A. & Larson, P. S. An unexpectedly high rate of revisions and removals in deep brain stimulation surgery: Analysis of multiple databases. Park. Relat. Disord. 2016; 33, 72–77.
3. Abosch, A., Yacoub, E., Ugurbil, K. & Harel, N. An assessment of current brain targets for deep brain stimulation surgery with susceptibility-weighted imaging at 7 Tesla. Neurosurgery, 2010; 67, 1745–1756.
4. Duchin, Y. et al. Patient-specific anatomical model for deep brain stimulation based on 7 Tesla MRI. PLoS One. 2018; 13, 1–23.
5. Teeuwisse, W. M., Brink, W. M. & Webb, A. G. Quantitative assessment of the effects of high-permittivity pads in 7 Tesla MRI of the brain. Magn. Reson. Med. 2012; 67, 1285–1293.
6. Schlemper, J. et al. Attention gated networks: Learning to leverage salient regions in medical images. Med. Image Anal. 2019; 53, 197–207.
7. Dai, J. et al. Deformable convolutional networks. Proc. IEEE Int. Conf. Comput. Vis. 2017; 764–773.
8. Ebani, E. J. et al. Improved targeting of the globus pallidus interna using quantitative susceptibility mapping prior to MR-guided focused ultrasound ablation in Parkinson’s disease. Clin. Imaging 2020; 68, 94–98.
9. Ewert, S. et al. Toward defining deep brain stimulation targets in MNI space: A subcortical atlas based on multimodal MRI, histology and structural connectivity. Neuroimage, 2018; 170, 271–282.
10. Pauli, W. M., Nili, A. N. & Michael T. J. Data descriptor: A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci. Data, 2018; 5, 1–13.
11. Xiao, Y. et al. A dataset of multi-contrast population-averaged brain MRI atlases of a Parkinson׳s disease cohort. Data Br. 2017; 12, 370–379.
12. He, X. et al. Disrupted basal ganglia-thalamocortical loops in focal to bilateral tonic-clonic seizures. Brain, 2020; 143, 175–190.
13. Wang, B. T. et al. Generation and evaluation of an ultra-high-field atlas with applications in DBS planning. Medical Imaging 2016: Image Processing, 2016; vol. 9784 97840H, SPIE.
14. Keuken, M. C. & Forstmann, B. U. A probabilistic atlas of the basal ganglia using 7 T MRI. Data Br. 2015; 4, 577–582.
Figures