3014
Neuromelanin Sensitive MRI and QSM of the Substantia Nigra in Parkinson’s-Linked Asian LRRK2 Carriers1National Neuroscience Institute, Singapore, Singapore, 2Duke-NUS Medical School, Singapore, Singapore, 3Lee Kong Chian School of Medicine, Nanyang Technological University, Singapore, Singapore, 4Singapore General Hospital, Singapore, Singapore, 5Singapore BioImaging Consortium, Singapore, Singapore, 6Seoul National University, Seoul, Korea, Republic of
Synopsis
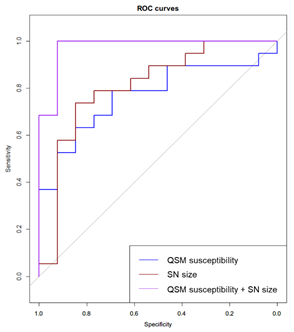
Asian-specific leucine-rich repeat kinase 2 (LRRK2) gene risk variants are associated with increased risk of idiopathic Parkinson’s disease (PD) and accelerated motor progression in disease. We examined the role of quantitative susceptibility mapping (QSM) and neuromelanin-sensitive MRI (NMS) in quantifying dopaminergic denervation in the substantia nigra (SN) in LRRK2 risk-carriers and non-carriers in PD. QSM susceptibility and high-iron area in the SN were significantly increased in PD risk-carriers compared to non-carriers; NMS showed no difference between these two groups. Combined quantitative QSM models displayed good classification performance discriminating risk-carrier from non-carrier groups (68.4% sensitivity, 92.3% specificity, AUC 0.804) in PD.
Introduction
Dopaminergic denervation in the substantia nigra (SN) occurs in idiopathic Parkinson’s disease (PD); and 5-10% of PD cases have a clear familial etiology.1 Mutations in the leucine-rich repeat kinase 2 (LRRK2) gene are the most common cause of autosomal dominant PD,2 and LRRK2 activity recently found to play a central role in PD pathogenesis.3 Asian-specific LRRKs gene risk variants have also been associated with increased risk of PD and accelerated motor progression in disease; but the pathophysiology of these4 is unknown. Neuromelanin-sensitive MRI (NMS) has been found to reliably quantify nigral damage in the SN and distinguish PD patients from healthy subjects.5,6 Pathological evidence also points to the important role of iron in neurodegeneration, possibly via toxic free radicals causing dopaminergic cell death.7 Iron-sensitive MRI techniques such as quantitative susceptibility mapping (QSM) have consistently found increased iron content in the SN in PD.8 High resolution susceptibility map-weighted images (SMWI) have improved the delineation of the SN on 3T imaging9 by utilizing susceptibility weighting mask derived from QSM to enhance the contrast for the SWI magnitude images. There is emerging MRI evidence of worse SN degeneration in genetic forms of PD.10-13 We hypothesize that QSM and NMS have a role in objective quantification of dopaminergic denervation in the SN in LRRK2 risk-carriers compared to non-carriers in PD patients.Methods
This study was approved by the local ethics board and informed consent obtained from all participants. PD patients clinically diagnosed by a movement disorders neurologist with more than two decades of experience, using the United Kingdom PD Brain Bank clinical criteria, were prospectively recruited from the clinic at a tertiary referral centre. All subjects underwent brain MRI scan on a 3T scanner and clinical motor assessment using the Unified Parkinson’s Disease Rating Scale motor subscore (UPDRS III) and Hoehn and Yahr staging (H&Y).The following high-resolution sequences were acquired, focussed over the midbrain: (1) 3D T2* SWI using a multi-echo gradient echo sequence with TR=48ms, TE=13.77/26.39/39ms, FA=20°, in-plane resolution=0.5x0.5x1mm3, number of slices = 32, scan duration 4:09mins; (2) NMS using a T1 TSE sequence with TR/TE=938/15 ms, voxel size=0.5x0.5x3mm3, number of slices=13, scan duration 10:25mins. QSM & SMWI images were reconstructed from the multi-echo GRE images using the SMWI software (Seoul National University, Seoul, South Korea).9 ROIs of the SN were manually drawn on SMWI and NMS images by a blinded rater.
Group comparisons on demographic and clinical variables were performed using chi-squared tests for categorical variables and two-tailed t-tests, or Kruskal-Wallis rank sum tests for continuous variables, as appropriate. Receiver operating characteristic (ROC) analysis was performed to determine the utility of the imaging measures in classifying patient groups and optimal cut-off points were selected to jointly maximize sensitivity and specificity. All p-values were two-tailed at the significance level of p<0.05. Multivariate analysis was performed after correcting for age, gender and disease duration. Statistical analysis was performed using R 4.0.3.
Results
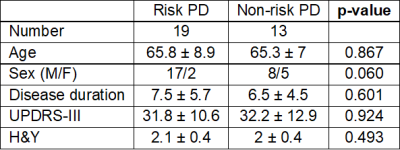
Nineteen PD LRRK2 risk-carriers and 13 non-carriers were recruited. Clinical demographics were reported in Figure 1. There were no significant differences in age, gender, disease duration, UPDRS-III or H&Y stage between the LRRK2 risk-carriers and non-carriers. Neither were SN ROI size or signal intensities derived from NMS different between carriers and non-carriers. QSM susceptibility values were significantly increased (p = 0.016) in LRRK2 risk-carriers (141 ± 33.97 ppb) compared to non-carriers (113.86 ± 21.55 ppb). QSM susceptibility displayed good classification performance in discriminating LRRK2 risk-carriers and non-carriers (78.95% sensitivity, 69.23% specificity, AUC = 0.769). Semi-automated segmentation on SMWI images after thresholding for voxels with high iron deposition (containing signal intensity 7 times of standard deviation less than background, Figure 2) showed the derived SN ROI size significantly larger (p = 0.016) in risk-carriers (91.08 ± 64.36 mm3) than non-carriers (32.77 ± 61.43 mm3). This derived SN ROI size also displayed good classification performance for distinguishing carriers and non-carriers (73.68% sensitivity, 84.62% specificity, AUC 0.8). Further combined quantitative QSM models based on SN ROI size and QSM susceptibility values further increased AUC slightly (0.804) with increased specificity (92.3%), at the cost of decreased sensitivity (68.4%) to differentiate carriers and non-carriers (Figure 3).Discussion
Studies evaluating the performance of MRI biomarkers in genetic PD is sparse, with small numbers of reports utilizing NMS. Ours is the first in LRRK2 risk-carriers using iron-sensitive MRI. Our NMS results concurred with previous studies reporting no significant differences between LRRK2 risk-carriers from non-carriers.10,11 On the other hand, QSM was able to distinguish LRRK2 risk-carriers from non-carriers, showing a larger SN containing higher iron deposition in LRRK2 risk-variant carriers. These results suggest that the NMS and QSM techniques interrogate and quantify differential aspects of the complex neurodegeneration in the SN.Conclusion
Iron-sensitive MRI techniques show potential as non-invasive surrogate biomarkers for quantifying differential dopaminergic neurodegeneration in the SN in LRRK2 risk-variant carriers in PD. Further studies using radiotracer imaging and ultra-high field MRI may further elucidate the interplay between neurodegeneration and iron deposition in the complex pathophysiology of PD.Acknowledgements
No acknowledgement found.References
1. Klein C, Westenberger A. Genetics of Parkinson's disease. Cold Spring Harb Perspect Med. 2012;2(1):a008888-a.
2. Zimprich A, Biskup S, Leitner P, et al. Mutations in LRRK2 cause autosomal-dominant parkinsonism with pleomorphic pathology. Neuron. 2004;44(4):601-7.
3. Di Maio R, Hoffman EK, Rocha EM, et al. LRRK2 activation in idiopathic Parkinson's disease. Science translational medicine. 2018;10(451):eaar5429.
4. Gopalai AA, Lim SY, Chua JY, et al. LRRK2 G2385R and R1628P mutations are associated with an increased risk of Parkinson's disease in the Malaysian population. Biomed Res Int. 2014;2014:867321.
5. Sasaki M, Shibata E, Tohyama K, et al. Neuromelanin magnetic resonance imaging of locus ceruleus and substantia nigra in Parkinson's disease. Neuroreport. 2006;17(11):1215-8.
6. Wang X, Zhang Y, Zhu C, et al. The diagnostic value of SNpc using NM-MRI in Parkinson's disease: meta-analysis. Neurol Sci. 2019;40(12):2479-89.
7. Dexter DT, Wells FR, Lees AJ, et al. Increased nigral iron content and alterations in other metal ions occurring in brain in Parkinson's disease. J Neurochem. 1989;52(6):1830-6.
8. Pyatigorskaya N, Sanz-Morère CB, Gaurav R, et al. Iron Imaging as a Diagnostic Tool for Parkinson's Disease: A Systematic Review and Meta-Analysis. Front Neurol. 2020;11:366.
9. Nam Y, Gho SM, Kim DH, et al. Imaging of nigrosome 1 in substantia nigra at 3T using multiecho susceptibility map-weighted imaging (SMWI). J Magn Reson Imaging. 2017;46(2):528-536.
10. Castellanos G, Fernández-Seara MA, Lorenzo-Betancor O, et al. Automated neuromelanin imaging as a diagnostic biomarker for Parkinson's disease. Mov Disord. 2015;30(7):945-52.
11. Correia Guedes L, Reimão S, Paulino P, et al. Neuromelanin magnetic resonance imaging of the substantia nigra in LRRK2-related Parkinson's disease. Mov Disord. 2017;32(9):1331-3.
12. Hatano T, Okuzumi A, Kamagata K, et al. Neuromelanin MRI is useful for monitoring motor complications in Parkinson's and PARK2 disease. J Neural Transm (Vienna). 2017;124(4):407-15.
13. Griffanti L, Klein JC, Szewczyk-Krolikowski K, et al. Cohort profile: the Oxford Parkinson's Disease Centre Discovery Cohort MRI substudy (OPDC-MRI). BMJ Open. 2020;10(8):e034110-e.
Figures