3012
Spatial changes of neuromelanin and iron content in substantial nigra pars compacta in early-stage idiopathic Parkinson’s disease1Radiology, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 2Medical Imaging Technology, 、School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China, 3Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China, 4Shanghai United Imaging Intelligence Co., Ltd., Shanghai, China, 5Medical Imaging Technology, School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China, 6Magnetic Resonance Innovations, Inc, Bingham Farms, MI, United States, 7Wayne State University, Detroit, MI, United States
Synopsis
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by progressive loss of neuromelanin-containing dopaminergic neurons and iron deposition mainly in the substantia nigra (SN). Previous studies mainly focused on global changes in both neuromelanin (NM) and iron content. In this study, we used a voxel-wise analysis to investigate the spatial changes of iron and NM content in PD and found that the ventral and medial part of the SN pars compacta had more iron deposition and less neuromelanin, especially, in early-stage PD.
Introduction
Parkinson’s disease (PD) is a common neurodegenerative disorder characterized by progressive loss of neuromelanin (NM) containing dopaminergic neurons mainly in the substantia nigra (SN)1 . The SN pars compacta (SNpc), the caudal part of the SN, contains 5 dense clusters of NM containing dopaminergic neurons (namely, Nigrosomes 1 to 5) and is mostly affected when PD is diagnosed2. In addition, iron accumulation has been verified along with the NM loss in SNpc3. These possibly pathological changes have been indirectly visualized in-vivo thanks to the development of NM and iron sensitive (via quantitative susceptibility mapping (QSM)) imaging. Previous studies have found that the signal intensity and size of the SN are both significantly reduced on NM imaging and increased iron deposition is seen in the lateral-dorsal part of the SNpc4,5. However, the spatial changes in the SNpc have not been fully investigated. Therefore, we explored spatial changes of iron and NM content in the SNpc via a voxel-wise analysis based on NM and QSM imaging.Methods
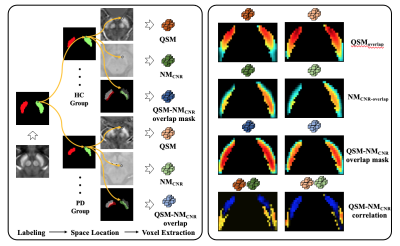
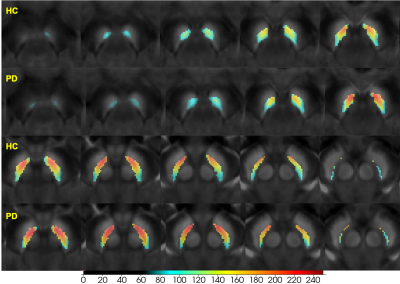
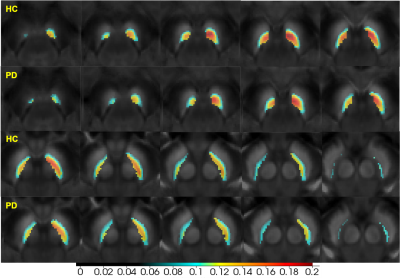
Forty (40) early-stage idiopathic PD and 40 age- and sex- matched healthy controls (HCs) were enrolled and scanned using a single 3D magnetization transfer contrast (MTC) gradient echo sequence at 3.0T. The SN and NM boundaries were manually drawn based on the QSM and NM sequences, respectively. The individual segmented SNs were transformed to a standard SN template derived and segmented from a 7.0 T QSM model6. SNpc was defined as the overlap of these two boundaries. The SNpc probability mask was then generated and thresholded at 0.34 and 0.36 for PD and HC, respectively (this represents the mean ratio of overlap of NM to the whole SN) (Figure 1). The contrast-to-noise (CNR) of the NM (NMCNR) image was calculated with reference to the adjacent cerebral peduncle. As these two image types were derived from the same sequence, a paired voxel-wise Pearson’s correlation analysis was done and a visualization map based on correlation coefficients was then generated.Results
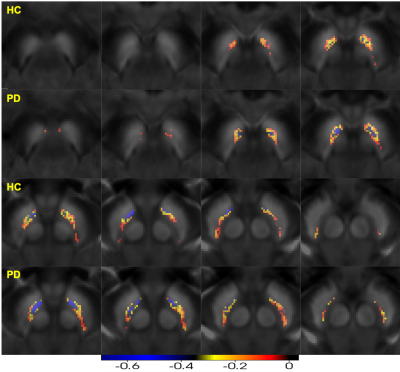
The susceptibility of the SNpc increased, while the NMCNR of SNpc decreased in early-stage PD, especially for the medial and ventral parts compared with the HCs (Figures 2,3). Further, paired negative correlation between NMCNR and QSM voxels was more frequently noticed in the ventral and medial part of SNpc. The negative correlation regions increased to include the caudal and dorsal parts of the SNpc in PD compared to those in HCs, and the moderate to high negative correlation regions (r<-0.4) of PD were larger than those of HCs (Figure 4).Discussion and Conclusion
Our preliminary results showed that the susceptibility reflecting the iron content of the SNpc increased and the NMCNR reflecting the NM content decreased in early-stage PD, which is consistent with previous studies4,5. Interestingly, this spatial change is prominent in the ventral and medial part of the SNpc, and there is a negative correlation between the increased iron content and decreased neuromelanin content, suggesting that the iron deposition is coincident with the NM containing neuron loss in this region during the pathological process of PD. The ventral and medial subregions of the SNpc might contain nigrosome 2 and nigrosome 4 according to a pathological and imaging control atlas7. The other subregions, especially, the dorsal and lateral part (presumably the nigrosome 1), have relatively less negative correlation, which suggests that there might be less iron accumulation in this subregion compared with that in the ventral and medial subregions of the SNpc for early-stage PD. In future work, it would be interesting to compare subregions with different correlations with clinical scores. In summary, iron deposition and NM containing neuron loss is prominent in the ventral and medial part of SNpc, especially, in early-stage PD. This region may correspond to nigrosome 2.Acknowledgements
We sincerely thank all the participants in this study.References
1. Zucca FA, Segura-Aguilar J, Ferrari E, et al. Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson's disease. Prog Neurobiol. 2017;155:96-119.
2. Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity. Brain. 1991;114 ( Pt 5):2283-2301.
3.Dexter DT, Wells FR, Agid F, et al. Increased nigral iron content in postmortem parkinsonian brain. Lancet. 1987;2(8569):1219-1220.
4. Langley J, Huddleston DE, Sedlacik J, Boelmans K, Hu XP. Parkinson's disease-related increase of T2*-weighted hypointensity in substantia nigra pars compacta. Mov Disord. 2017;32(3):441-449.
5. Huddleston DE, Langley J, Sedlacik J, Boelmans K, Factor SA, Hu XP. In vivo detection of lateral-ventral tier nigral degeneration in Parkinson's disease. Hum Brain Mapp. 2017;38(5):2627-2634.
6. Bollmann S, Andrew Janke, Lars Marstaller, David Reutens, Kieran O’Brien, and Markus Barth. GRE and QSM Average 7T Model. 2017.7. Massey LA, Miranda MA, Al-Helli O, et al. 9.4T MR microscopy of the substantia nigra with pathological validation in controls and disease. Neuroimage Clin. 2017;13:154-163.
Figures