3009
The presumed structure alterations of Spinocerebellar Ataxias 3: from presymptomatic to the symptomatic stage1Sun Yat-sen University, Guangzhou, China, 2Simens Healthcare, Guangzhou, China
Synopsis
There is an evolving history of structural images with SCA3 patients in different disease stages. The WM damage starts with the impairment of ICP and goes through SCP extends to the midbrain, then widespread to the whole brain. The alteration of GMV does not occur until the arise of ataxia symptom, then began to involve the medulla, cerebellum, and pons, and developed to involve basal ganglion, finally affect the cortical cortex. The impairment of WM tracts precedes the GM atrophy and, irrespective of the patients with or without clinical manifestation, the identified WM damage was significantly correlated with SARA.
Introduction
Spinocerebellar ataxia type3 (SCA3) is an autosomal dominantly inherited disease caused by the abnormal expansion of cytosine-adenine-guanine (CAG) triplet repeats in encoding region of the ATXN3 gene. Currently, a handful of therapeutic options are showing great prospects in treatments for fatal SCA3. However, efficient laboratorial, clinical and imaging markers for fully track SCA3, from its preataxic phase to the late stage are still in lack, moreover, the candidates for surrogate biomarkers of the presymptomatic stage were even rarer described in the literatures. Besides, compared with clinical biomarkers, the imaging biomarkers showed high effect sizes and were more suitable for the RCT study and was most studied in the literatures. However, there were few brain structure researches on presymptomatic SCA3 patients, and the results among studies were variable. And the brain structural alterations of SCA3 in the pre-symptomatic and symptomatic stage and how the structure changes during the follow-up in the Asian group are still in need. Further, past studies all use DTI model to investigate the white matter (WM) damage of SCA3. DKI, though has been proved to be more advanced in evaluation and diagnosis in various diseases, hasn’t been used in evaluating SCA3. Thus, our research was to investigate the WM and gray matter (GM) alterations of spinocerebellar ataxia type 3 (SCA3) patients, from presymptomatic to the symptomatic stage, and to analyze the correlations of these alterations with disease severity.Method
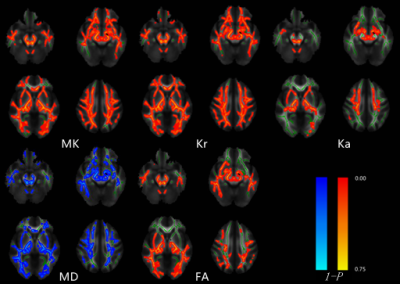
Prospectively enrolled 43 gene confirmed SCA3 patients (37 symptomatic (23 female, mean age 40.2y) and 15 presymptomatic (6 female, mean age 29.1y) patients) and 35 healthy controls (HCs) (22F, mean age 38.5y) with similar age, sex and BMI in our study. All cases were referred to whole brain DKI and T1mprage scanning. Both difference of gray matter volume (GMV) and DKI parameters map including mean kurtosis (MK), axial kurtosis (Ka), radial kurtosis (Kr), mean diffusion (MD) and fractional anisotropy (FA) were analyzed via VBM and TBSS methods. The correlations among the WM and GM alterations and the score of the assessment and rating of ataxia (SARA) were analyzed by spearman analysis.Results
Compared to HCs, pre-symptomatic patients showed WM microstructural abnormalities mainly in bilateral cerebellar inferior peduncles (ICP), cerebral peduncles, posterior lambs of internal capsule and medial lemniscus. Whereas, there was no significant GMV difference was found. Meanwhile, the symptomatic patients, compared with HCs, showed diffusely WM damaged (P<0.05, TFCE correction) and had significantly reduced GMV in cerebellum, pons, medulla, bilateral putamen, lentiform nucleus and significantly increased GMV in the bilateral thalamus (P< 0.05, FWE correction). Besides, compared to pre-symptomatic SCA3, symptomatic SCA3 showed WM damage in bilateral superior cerebellar peduncles (SCP), cerebral peduncles, posterior lambs of internal capsule, right posterior thalamic radiation (include optic radiation) and superior corona radiata(P<0.05,TFCE correction)and had significantly reduced GMV in pons. In addition, there was significant negative correlation between DKI parameters and SARA scores in WM abnormalities of SCA3 patients with or without clinical manifestation(P<0.05,TFCE correction) and, unexpectedly, the SARA score was not significantly correlated with GMV (P> 0.05, FWE correction).conclusion
Our study indicates that there is an evolving history of structural images with SCA3 patients in different disease stages. The WM damage starts with the impairment of ICP and goes through SCP extends to the midbrain, then widespread to the whole brain. The alteration of GMV does not occur until the arise of ataxia symptom, then began to involve the medulla, cerebellum, and pons, and developed to involve basal ganglion, after the decompensation of bilateral dorsal thalamus, finally affect the cortical cortex. The impairment of WM tracts precedes the GM atrophy and, irrespective of the patients with or without clinical manifestation, the identified WM damage was significantly correlated with SARA.Acknowledgements
Nothing to declare.References
1. Costa MDC Recent therapeutic prospects for Machado-Joseph disease. Curr Opin Neurol. 2020;33(4):519-26.
2. Furtado GV, Oliveira CM, Bolzan G, et al. State biomarkers for Machado Joseph disease: Validation, feasibility and responsiveness to change. Genet Mol Biol. 2019;42(1 suppl 1):238-51.
3. Adanyeguh IM, Perlbarg V, Henry P-G, et al. Autosomal dominant cerebellar ataxias: Imaging biomarkers with high effect sizes. NeuroImage: Clinical. 2018;19(858-67.
4. Jacobi H, Reetz K, du Montcel ST, et al. Biological and clinical characteristics of individuals at risk for spinocerebellar ataxia types 1, 2, 3, and 6 in the longitudinal RISCA study: analysis of baseline data. The Lancet Neurology. 2013;12(7):650-8.
5. Joers JM, Deelchand DK, Lyu T, et al. Neurochemical abnormalities in premanifest and early spinocerebellar ataxias. Ann Neurol. 2018;83(4):816-29.
6. Rezende TJR, de Paiva JLR, Martinez ARM, et al. Structural signature of SCA3: From presymptomatic to late disease stages. Annals of Neurology. 2018;84(3):401-8.
7. Wu X, Liao X, Zhan Y, et al. Microstructural Alterations in Asymptomatic and Symptomatic Patients with Spinocerebellar Ataxia Type 3: A Tract-Based Spatial Statistics Study. Front Neurol. 2017;8(714.
8. de Mattos EP, Leotti VB, Soong BW, et al. Age at onset prediction in spinocerebellar ataxia type 3 changes according to population of origin. Eur J Neurol. 2019;26(1):113-20.
9. Arruda WO, Meira AT, Ono SE, et al. Volumetric MRI Changes in Spinocerebellar Ataxia (SCA3 and SCA10) Patients. Cerebellum. 2020;19(4):536-43.
10. D'Abreu A, Franca MC, Jr., Yasuda CL, et al. Neocortical atrophy in Machado-Joseph disease: a longitudinal neuroimaging study. J Neuroimaging. 2012;22(3):285-91.
11. Akcimen F, Ross JP, Liao C, et al. Expanded CAG Repeats in ATXN1, ATXN2, ATXN3, and HTT in the 1000 Genomes Project. Mov Disord. 2020.
12. Gan SR, Figueroa KP, Xu HL, et al. The impact of ethnicity on the clinical presentations of spinocerebellar ataxia type 3. Parkinsonism Relat Disord. 2020;72(37-43.
13. Duarte JV, Faustino R, Lobo M, et al. Parametric fMRI of paced motor responses uncovers novel whole-brain imaging biomarkers in spinocerebellar ataxia type 3. Hum Brain Mapp. 2016;37(10):3656-68.
14. Wang PS, Wu YT, Wang TY, et al. Supratentorial and Infratentorial Lesions in Spinocerebellar Ataxia Type 3. Front Neurol. 2020;11(124.
15. Hernandez-Castillo CR, Diaz R, Campos-Romo A, et al. Neural correlates of ataxia severity in spinocerebellar ataxia type 3/Machado-Joseph disease. Cerebellum Ataxias. 2017;4(7.
16. de Rezende TJ, D'Abreu A, Guimaraes RP, et al. Cerebral cortex involvement in Machado-Joseph disease. Eur J Neurol. 2015;22(2):277-83, e23-4.
17. Park YW, Joers JM, Guo B, et al. Assessment of Cerebral and Cerebellar White Matter Microstructure in Spinocerebellar Ataxias 1, 2, 3, and 6 Using Diffusion MRI. Front Neurol. 2020;11(411.