3005
The nomogram of MRI-based radiomics with complementary visual features by machine learning improves stratification of glioblastoma patients1Zhejiang Provincial People’s Hospital, Hangzhou, China, 2MR Research, GE healthcare (China), SHANG HAI, China
Synopsis
This preliminary study explored the application of radiomics MRI in overall survival(OS) of glioblastoma patients. We found that EPI, age, and radiomic signature are independent predictors of OS for glioblastoma patients. The nomogram was created by integrating the three independent predictors, had the best performance when stratifying glioblastoma patients into long- versus short-term survival, which could help clinicians develop optimal treatment plans.
Synopsis
This preliminary study explored the application of radiomics MRI in overall survival(OS) of glioblastoma patients.In addition, the independent predictors of OS was analyzed, and a prediction radiomics nomogram based on independent predictors was constructed. We found that EPI, age, and radiomic signature are independent predictors of OS for glioblastoma patients. The nomogram was created by integrating the three independent predictors, had the best performance when stratifying glioblastoma patients into long- versus short-term survival, which could help clinicians develop optimal treatment plans.Purpose
Radiomics was performed for preoperative magnetic resonance imaging (MRI) studies of glioblastoma (GBM) patients to determine the prognosis of GBM patients and proved to be robust. Ependymal and/or pia mater involvement (EPI) was considered to affect overall survival (OS). However, radiomics employed for an automatic or manually segmented tumor could not reflect EPI involvement. Whether a prediction model based on a combination of radiomics and EPI can better predict OS has not yet been studied. The purpose of this study was construct a radiomics nomogram to stratify GBM patients into long- versus short-term survival by machine learning using multiparametric MRI-based radiomic features and specific visual features as EPI.Methods
Patient data from BRATS2018 and local hospital were retrospectively analyzed. All images were assessed for EPI and multifocality. All tumor tissues were fully automatically segmented from multiparametric MRI and classified into three subregions to calculate the radiomic features. The most powerful radiomic features were selected to constitute radiomic signature. Then, the prediction models featuring independent predictors were created using machine-learning methods to select the optimal model, and a nomogram was built to stratify GBM patients into the long- or short-term OS groups.Results
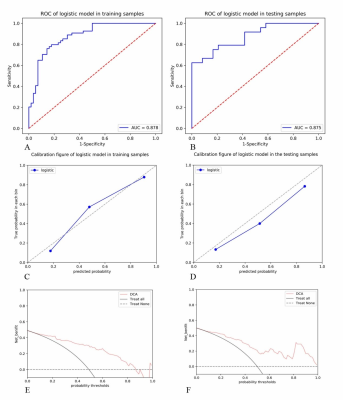
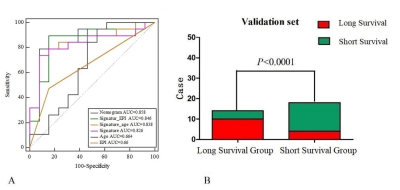
The nomogram had a survival prediction accuracy of 0.878 and 0.875, a specificity of 0.875 and 0.583, and a sensitivity of 0.704 and 0.833, respectively, in the training and test set (Fig. 1). The ROC curve showed the accuracy of the nomogram, radiomic signature, age, and EPI for external validation set were 0.858, 0.826, 0.664and 0.66 in the validate set, respectively (Fig. 2).Discussion and Conclusion
This study demonstrates that in a radiomics nomogram integrated with a radiomic signature, complementary visual features such as EPI and age were found to be robust for the stratification of GBM patients into long- versus short-term survival and would be pragmatic in clinic practice.Acknowledgements
NoneReferences
[1] Bakas S, Akbari H, Sotiras A, Bilello M, Rozycki M, Kirby JS, et al. Advancing The Cancer Genome Atlas glioma MRI collections with expert segmentation labels and radiomic features. Sci Data. (2017) 4:170117.
[2] Bakas S., Reyes M., Jakab A., Bauer S., Rempfler M., Crimi A., et al. Identifying the best machine learning algorithms for brain tumor segmentation, progression assessment, and overall survival prediction in the BRATS challenge. (2018 )arXiv:1811.02629v1
Figures